Plasma neurofilament light chain in children with relapsing MS receiving teriflunomide or placebo: A post hoc analysis of the randomized TERIKIDS trial
- PMID: 36632983
- PMCID: PMC9972233
- DOI: 10.1177/13524585221144742
Plasma neurofilament light chain in children with relapsing MS receiving teriflunomide or placebo: A post hoc analysis of the randomized TERIKIDS trial
Abstract
Background: The phase 3 TERIKIDS study demonstrated efficacy and manageable safety for teriflunomide versus placebo in children with relapsing multiple sclerosis (RMS).
Objective: Evaluate plasma neurofilament light chain (pNfL) concentrations in TERIKIDS.
Methods: Patients received placebo or teriflunomide (14 mg adult equivalent) for up to 96 weeks in the double-blind (DB) period. In the open-label extension (OLE), all patients received teriflunomide until up to 192 weeks after randomization. pNfL was measured using single-molecule array assay (Simoa® NF-light™).
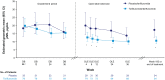
Results: Baseline mean age was 14.5 years; 69.4% were female. Baseline geometric least square mean pNfL levels were similar for teriflunomide (n = 78) and placebo (n = 33) patients (19.83 vs 18.30 pg/mL). Over the combined DB and OLE periods, pNfL values were lower for teriflunomide versus placebo (analysis of variance p < 0.01; Week 192: 10.61 vs 17.32 pg/mL). Observed between-group pNfL differences were attenuated upon adjustment for gadolinium (Gd)-enhancing or new/enlarged T2 lesion counts at DB Week 24. Higher baseline pNfL levels were associated with shorter time since first MS symptom onset, higher baseline Gd-enhancing lesion counts and T2 lesion volume, and increased hazard of high magnetic resonance imaging activity or clinical relapse during the DB period.
Conclusion: Teriflunomide treatment was associated with significantly reduced pNfL levels in children with RMS.
Clinicaltrials.gov identifier: NCT02201108.
Keywords: Neurofilament light chain; pediatric MS; teriflunomide.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article:
Figures

Comment in
-
The contribution of neurofilament light chain to better characterize pediatric multiple sclerosis (editorial on: Plasma neurofilament light chain in children with relapsing MS receiving teriflunomide or placebo: A post hoc analysis of the randomized TERIKIDS trial).Mult Scler. 2023 May;29(6):668-670. doi: 10.1177/13524585231161148. Epub 2023 Mar 23. Mult Scler. 2023. PMID: 36960483 No abstract available.
References
-
- Ness JM, Chabas D, Sadovnick AD, et al. Clinical features of children and adolescents with multiple sclerosis. Neurology 2007; 68: S37–S45. - PubMed
-
- Renoux C, Vukusic S, Mikaeloff Y, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med 2007; 356: 2603–2613. - PubMed
-
- Krysko KM, Graves JS, Rensel M, et al. Real-world effectiveness of initial disease-modifying therapies in pediatric multiple sclerosis. Ann Neurol 2020; 88(1): 42–55. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

