Scavenger receptor a is a major homeostatic regulator that restrains drug-induced liver injury
- PMID: 36632993
- PMCID: PMC10410742
- DOI: 10.1097/HEP.0000000000000044
Scavenger receptor a is a major homeostatic regulator that restrains drug-induced liver injury
Abstract
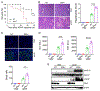
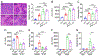
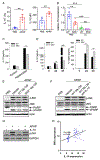
Background and aim: Drug-induced liver injury occurs frequently and can be life threatening. Although drug-induced liver injury is mainly caused by the direct drug cytotoxicity, increasing evidence suggests that the interplay between hepatocytes and immune cells can define this pathogenic process. Here, we interrogate the role of the pattern recognition scavenger receptor A (SRA) for regulating hepatic inflammation and drug-induced liver injury.
Approach and results: Using acetaminophen (APAP) or halothane-induced liver injury models, we showed that SRA loss renders mice highly susceptible to drug hepatotoxicity, indicated by the increased mortality and liver pathology. Mechanistic studies revealed that APAP-induced liver injury exaggerated in the absence of SRA was associated with the decreased anti-inflammatory and prosurvival cytokine IL-10 concomitant with excessive hepatic inflammation. The similar correlation between SRA and IL-10 expression was also seen in human following APAP uptake. Bone marrow reconstitution and liposomal clodronate depletion studies established that the hepatoprotective activity of SRA mostly resized in the immune sentinel KCs. Furthermore, SRA-facilitated IL-10 production by KCs in response to injured hepatocytes mitigated activation of the Jun N-terminal kinase-mediated signaling pathway in hepatocytes. In addition, supplemental use of IL-10 with N -acetylcysteine, only approved treatment of APAP overdose, conferred mice improved protection from APAP-induced liver injury.
Conclusion: We identify a novel hepatocyte-extrinsic pathway governed by the immune receptor SRA that maintains liver homeostasis upon drug insult. Giving that drug (ie, APAP) overdose is the leading cause of acute liver failure, targeting this hepatoprotective SRA-IL-10 axis may provide new opportunities to optimize the current management of drug-induced liver injury.
Copyright © 2023 American Association for the Study of Liver Diseases.
Conflict of interest statement
Arun J. Sanyal is President of Sanyal Biotechnology and has stock options in Genfit, Akarna, Tiziana, Indalo, Durect and Galmed. He has served as a consultant to Astra Zeneca, Nitto Denko, Enyo, Ardelyx, Conatus, Nimbus, Amarin, Salix, Tobira, Takeda, Jannsen, Gilead, Terns, Birdrock, Merck, Valeant, Boehringer-Ingelheim, Lilly, Hemoshear, Zafgen, Novartis, Novo Nordisk, Pfizer, Exhalenz and Genfit. He has been an unpaid consultant to Intercept, Echosens, Immuron, Galectin, Fractyl, Syntlogic, Affimune, Chemomab, Zydus, Nordic Bioscience, Albireo, Prosciento, Surrozen, and Bristol Myers Squibb. His institution has received grant support from Gilead, Salix, Tobira, Bristol Myers, Shire, Intercept, Merck, Astra Zeneca, Malinckrodt, Cumberland, and Norvatis. He receives royalties from Elsevier and UptoDate.
Figures

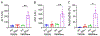


References
-
- Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349:474–85. - PubMed
-
- Andrade RJ, Chalasani N, Bjornsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB, et al. Drug-induced liver injury. Nat Rev Dis Primers. 2019;5:58. - PubMed
-
- Larsen FS, Wendon J. Understanding paracetamol-induced liver failure. Intensive Care Med. 2014;40:888–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

