Clinical outcomes of second‑line chemotherapy after gemcitabine and cisplatin plus S‑1 treatment for patients with advanced biliary tract cancer in the KHBO1401‑3A study
- PMID: 36633148
- PMCID: PMC9868686
- DOI: 10.3892/or.2023.8478
Clinical outcomes of second‑line chemotherapy after gemcitabine and cisplatin plus S‑1 treatment for patients with advanced biliary tract cancer in the KHBO1401‑3A study
Abstract
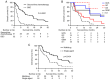
Since the completion of the KHBO1401 study, which evaluated the efficacy of the combination of gemcitabine (GEM) and cisplatin (GC) compared with GC plus S‑1 (GCS), GCS has become a standard chemotherapy for patients with advanced biliary tract cancer (BTC). However, there are currently no data revealing second‑line therapy options after GCS. The present study aimed to evaluate the survival outcomes of patients receiving second‑line chemotherapy for advanced BTC, refractory or intolerant to GCS, using data from the KHBO1401 study. Patients who received a second‑line treatment after GCS chemotherapy between July 2014 and February 2016 were retrospectively studied. Overall survival (OS) was calculated from the day of GCS treatment failure or the first day of second‑line chemotherapy to the final follow‑up date or until death from any cause. Among 83 patients refractory or intolerant to GCS chemotherapy, 51 (61%) received second‑line chemotherapy, including GCS (n=8), GC (n=15), GEM (n=6), GEM plus S‑1 (GS) (n=4) and S‑1 (n=18). The 6‑ and 12‑month OS rates were 66.7 and 44.4%, respectively, following second‑line chemotherapy, and 6.3 and 3.1%, respectively, in the best supportive care group (P<0.0001). In addition, the 12‑ and 24‑month OS rates were 59.3 and 36.2%, respectively, in the multidrug chemotherapy group, and 26.9 and 9.0%, respectively, in the single‑agent chemotherapy group (P=0.0191). These results suggested that second‑line combination chemotherapy is a viable treatment option for patients with advanced BTC that is refractory or intolerant to first‑line GCS therapy.
Keywords: S‑1; biliary tract cancer; cisplatin; gemcitabine; second‑line.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Morizane C, Okusaka T, Mizusawa J, Katayama H, Ueno M, Ikeda M, Ozaka M, Okano N, Sugimori K, Fukutomi A, et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: The FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol. 2019;30:1950–1958. doi: 10.1093/annonc/mdz402. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

