Early treatment with terlipressin in patients with hepatorenal syndrome yields improved clinical outcomes in North American studies
- PMID: 36633470
- PMCID: PMC9827960
- DOI: 10.1097/01.HC9.0000897228.91307.0c
Early treatment with terlipressin in patients with hepatorenal syndrome yields improved clinical outcomes in North American studies
Abstract
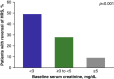
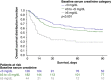
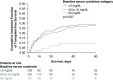
Hepatorenal syndrome type 1 (HRS-1) is a serious complication of advanced cirrhosis and a potentially reversible form of acute kidney injury that is associated with rapidly deteriorating kidney function. Liver transplantation remains the only curative treatment for decompensated cirrhosis. However, terlipressin, a vasopressin analog, successfully reverses HRS-1, and may improve patient survival while awaiting liver transplantation. Patients with higher baseline serum creatinine have a reduced response to treatment with terlipressin. These post hoc analyses examined pooled data from 352 patients with HRS-1 treated with terlipressin in 3 North American-centric, Phase III, placebo-controlled clinical studies (i.e. OT-0401, REVERSE, and CONFIRM)-across 3 serum creatinine subgroups (i.e. <3, ≥3-<5, and ≥5 mg/dL)-to further delineate their correlation with HRS reversal, renal replacement therapy-free survival, and overall survival. Serum creatinine was significantly associated with HRS reversal in univariate and multivariate logistic regression analyses (P<0.001). The incidence of HRS reversal inversely correlated with serum creatinine subgroup (<3 mg/dL, 49.2%; ≥3-<5 mg/dL, 28.0%; ≥5 mg/dL, 9.1%). At Day 30 follow-up, renal replacement therapy-free survival was significantly higher for patients with HRS-1 in the lower serum creatinine subgroups than in the higher subgroup (<5 vs. >5 mg/dL; p=0.01). Terlipressin-treated patients with HRS-1, with a lower baseline serum creatinine level, had a higher overall survival (p<0.001) and higher transplant-free survival at Day 90 (p=0.04). Patients with HRS-1 and lower serum creatinine levels who were treated with terlipressin had higher HRS reversal and survival outcomes, highlighting the significant need to identify and treat patients with HRS-1 early when they often have lower serum creatinine levels, and likely a greater response to terlipressin.
Trial registration: ClinicalTrials.gov NCT02770716 NCT00089570 NCT01143246.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
M.P.C. reports consultant fees and grants from Mallinckrodt Pharmaceuticals during the conduct of the studies, and grants from Gilead and Sonic Incytes, outside the submitted work. H.E.V. reports consultation fees and grants received from Mallinckrodt during the conduct of this study. He received grants from Ocelot and Sequana. A.S.B. reports grants received from Mallinckrodt for the conduct of these clinical studies and Exact Sciences for the conduct of a clinical trial. N.T.P. reports grants received from Mallinckrodt for the conduct of the study. He also report grants from Grifols, intercept, cytosorbents, durect, and Salix. V.R.P. reports receiving no grants or consultation fees for the conduct of these clinical studies. K.J. holds intellectual property rights with Mallinckrodt Pharmaceuticals.
Figures

References
-
- Angeli P, Garcia-Tsao G, Nadim MK, Parikh CR. News in pathophysiology, definition and classification of hepatorenal syndrome: a step beyond the International Club of Ascites (ICA) consensus document. J Hepatol. 2019;71:811–22. - PubMed
-
- Boyer TD, Sanyal AJ, Wong F, Frederick RT, Lake JR, O’Leary JG, et al. . Terlipressin plus albumin is more effective than albumin alone in improving renal function in patients with cirrhosis and hepatorenal syndrome type 1. Gastroenterology. 2016;150:1579–89. e2. - PubMed
-
- Angeli P, Ginès P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. . Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62:968–74. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

