Defining the Optimal Duration of Therapy for Hospitalized Patients With Complicated Urinary Tract Infections and Associated Bacteremia
- PMID: 36633559
- PMCID: PMC10411929
- DOI: 10.1093/cid/ciad009
Defining the Optimal Duration of Therapy for Hospitalized Patients With Complicated Urinary Tract Infections and Associated Bacteremia
Abstract
Background: Limited data are available to guide effective antibiotic durations for hospitalized patients with complicated urinary tract infections (cUTIs).
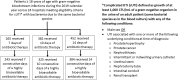
Methods: We conducted an observational study of patients ≥18 years at 24 US hospitals to identify the optimal treatment duration for patients with cUTI. To increase the likelihood patients experienced true infection, eligibility was limited to those with associated bacteremia. Propensity scores were generated for an inverse probability of treatment weighted analysis. The primary outcome was recurrent infection with the same species ≤30 days of completing therapy.
Results: 1099 patients met eligibility criteria and received 7 (n = 265), 10 (n = 382), or 14 (n = 452) days of therapy. There was no difference in the odds of recurrent infection for patients receiving 10 days and those receiving 14 days of therapy (aOR: .99; 95% CI: .52-1.87). Increased odds of recurrence was observed in patients receiving 7 days versus 14 days of treatment (aOR: 2.54; 95% CI: 1.40-4.60). When limiting the 7-day versus 14-day analysis to the 627 patients who remained on intravenous beta-lactam therapy or were transitioned to highly bioavailable oral agents, differences in outcomes no longer persisted (aOR: .76; 95% CI: .38-1.52). Of 76 patients with recurrent infections, 2 (11%), 2 (10%), and 10 (36%) in the 7-, 10-, and 14-day groups, respectively, had drug-resistant infections (P = .10).
Conclusions: Seven days of antibiotics appears effective for hospitalized patients with cUTI when antibiotics with comparable intravenous and oral bioavailability are administered; 10 days may be needed for all other patients.
Keywords: E. coli; UTI; antibiotics; duration; gram-negative bacteremia.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. S. E. C. reports receiving personal fees from Basilea and Theravance, outside of the submitted work, including participation on a Data Safety Monitoring Board or Advisory Board for Debiopharm. E. L. H. reports consulting fees from Wolters-Kluwer (Lexi-Comp). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

Comment in
-
Taking on the Fundamental Questions in Infectious Diseases Two-at-a-Time.Clin Infect Dis. 2023 May 3;76(9):1613-1614. doi: 10.1093/cid/ciad013. Clin Infect Dis. 2023. PMID: 36631160 No abstract available.
-
Optimal Duration of Therapy for Inpatient Complicated Urinary Tract Infections Remains Undefined.Clin Infect Dis. 2023 Aug 14;77(3):496-497. doi: 10.1093/cid/ciad208. Clin Infect Dis. 2023. PMID: 37021690 No abstract available.
Similar articles
-
Effectiveness of Shorter Versus Longer Durations of Therapy for Common Inpatient Infections Associated With Bacteremia: A Multicenter, Propensity-Weighted Cohort Study.Clin Infect Dis. 2020 Dec 15;71(12):3071-3078. doi: 10.1093/cid/ciz1197. Clin Infect Dis. 2020. PMID: 31858136
-
Oral Antibiotics for Treatment of Gram-Negative Bacteremia in Solid Organ Transplant Recipients: A Propensity Score Weighted Retrospective Observational Study.Clin Infect Dis. 2024 Jul 19;79(1):208-214. doi: 10.1093/cid/ciae007. Clin Infect Dis. 2024. PMID: 38195100
-
Intravenous-only or Intravenous Transitioned to Oral Antimicrobials for Enterobacteriaceae-Associated Bacteremic Urinary Tract Infection.Pharmacotherapy. 2017 Nov;37(11):1479-1483. doi: 10.1002/phar.2024. Epub 2017 Oct 23. Pharmacotherapy. 2017. PMID: 28869655 Free PMC article.
-
Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.Drugs. 2022 Apr;82(5):533-557. doi: 10.1007/s40265-022-01688-1. Epub 2022 Mar 16. Drugs. 2022. PMID: 35294769 Review.
-
Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli.Dan Med Bull. 2011 Apr;58(4):B4187. Dan Med Bull. 2011. PMID: 21466767 Review.
Cited by
-
Oral β-Lactams, Fluoroquinolones, or Trimethoprim-Sulfamethoxazole for Definitive Treatment of Uncomplicated Escherichia coli or Klebsiella Species Bacteremia From a Urinary Tract Source.Open Forum Infect Dis. 2023 Dec 27;11(2):ofad657. doi: 10.1093/ofid/ofad657. eCollection 2024 Feb. Open Forum Infect Dis. 2023. PMID: 38370295 Free PMC article. Clinical Trial.
-
Antibiotic Therapy Duration for Multidrug-Resistant Gram-Negative Bacterial Infections: An Evidence-Based Review.Int J Mol Sci. 2025 Jul 18;26(14):6905. doi: 10.3390/ijms26146905. Int J Mol Sci. 2025. PMID: 40725151 Free PMC article. Review.
-
An investigation of broad-spectrum antibiotic-induced liver injury based on the FDA Adverse Event Reporting System and retrospective observational study.Sci Rep. 2024 Aug 6;14(1):18221. doi: 10.1038/s41598-024-69279-6. Sci Rep. 2024. PMID: 39107511 Free PMC article.
-
Oral Antibiotics for Bacteremia and Infective Endocarditis: Current Evidence and Future Perspectives.Microorganisms. 2023 Dec 18;11(12):3004. doi: 10.3390/microorganisms11123004. Microorganisms. 2023. PMID: 38138148 Free PMC article. Review.
-
Evaluation of Short Versus Long Courses of Antibiotics in Critically Ill Patients With Gram-Negative Bloodstream Infections.Ann Pharmacother. 2024 Nov;58(11):1081-1088. doi: 10.1177/10600280241231611. Epub 2024 Feb 12. Ann Pharmacother. 2024. PMID: 38347703
References
-
- Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:625–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

