Intranasal Carbetocin Reduces Hyperphagia, Anxiousness, and Distress in Prader-Willi Syndrome: CARE-PWS Phase 3 Trial
- PMID: 36633570
- PMCID: PMC10271225
- DOI: 10.1210/clinem/dgad015
Intranasal Carbetocin Reduces Hyperphagia, Anxiousness, and Distress in Prader-Willi Syndrome: CARE-PWS Phase 3 Trial
Abstract
Context: Prader-Willi syndrome (PWS) is a rare genetic disorder characterized by endocrine and neuropsychiatric problems including hyperphagia, anxiousness, and distress. Intranasal carbetocin, an oxytocin analog, was investigated as a selective oxytocin replacement therapy.
Objective: To evaluate safety and efficacy of intranasal carbetocin in PWS.
Design: Randomized, double-blind, placebo-controlled phase 3 trial with long-term follow-up.
Setting: Twenty-four ambulatory clinics at academic medical centers.
Participants: A total of 130 participants with PWS aged 7 to 18 years.
Interventions: Participants were randomized to 9.6 mg/dose carbetocin, 3.2 mg/dose carbetocin, or placebo 3 times daily during an 8-week placebo-controlled period (PCP). During a subsequent 56-week long-term follow-up period, placebo participants were randomly assigned to 9.6 mg or 3.2 mg carbetocin, with carbetocin participants continuing at their previous dose.
Main outcome measures: Primary endpoints assessed change in hyperphagia (Hyperphagia Questionnaire for Clinical Trials [HQ-CT]) and obsessive-compulsive symptoms (Children's Yale-Brown Obsessive-Compulsive Scale [CY-BOCS]) during the PCP for 9.6 mg vs placebo, and the first secondary endpoints assessed these same outcomes for 3.2 mg vs placebo. Additional secondary endpoints included assessments of anxiousness and distress behaviors (PWS Anxiousness and Distress Behaviors Questionnaire [PADQ]) and clinical global impression of change (CGI-C).
Results: Because of onset of the COVID-19 pandemic, enrollment was stopped prematurely. The primary endpoints showed numeric improvements in both HQ-CT and CY-BOCS which were not statistically significant; however, the 3.2-mg arm showed nominally significant improvements in HQ-CT, PADQ, and CGI-C scores vs placebo. Improvements were sustained in the long-term follow-up period. The most common adverse event during the PCP was mild to moderate flushing.
Conclusions: Carbetocin was well tolerated, and the 3.2-mg dose was associated with clinically meaningful improvements in hyperphagia and anxiousness and distress behaviors in participants with PWS.
Clinical trials registration number: NCT03649477.
Keywords: Prader-Willi syndrome; anxiety; carbetocin; hyperphagia; oxytocin; vasopressin.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

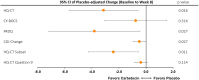
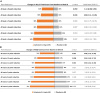

Comment in
-
New Avenues for Pharmacological Management of Hyperphagia and Associated Behavioral Disorders in Prader-Willi Syndrome.J Clin Endocrinol Metab. 2023 Aug 18;108(9):e895-e896. doi: 10.1210/clinem/dgad131. J Clin Endocrinol Metab. 2023. PMID: 36896885 No abstract available.
References
-
- Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14(1):10‐26. - PubMed
-
- Driscoll DJ, Miller JL, Schwartz S, Cassidy SB. Prader-Willi syndrome. In: Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, et al., eds. GeneReviews((R)) University of Washington; 1993:1‐37.

