Two-Electron Mixed Valency in a Heterotrimetallic Nickel-Vanadium-Nickel Complex
- PMID: 36633592
- PMCID: PMC9890480
- DOI: 10.1021/acs.inorgchem.2c03381
Two-Electron Mixed Valency in a Heterotrimetallic Nickel-Vanadium-Nickel Complex
Abstract
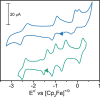
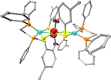
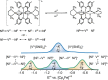
Mixed-valence complexes represent an enticing class of coordination compounds to interrogate electron transfer confined within a molecular framework. The diamagnetic heterotrimetallic anion, [V(SNS)2{Ni(dppe)}2]-, was prepared by reducing (dppe)NiCl2 in the presence of the chelating metalloligand [V(SNS)2]- [dppe = bis(diphenylphosphino)ethane; (SNS)3- = bis(2-thiolato-4-methylphenyl)amide]. Vanadium-nickel bonds span the heterotrimetallic core in the structure of [V(SNS)2{Ni(dppe)}2]-, with V-Ni bond lengths of 2.78 and 2.79 Å. One-electron oxidation of monoanionic [V(SNS)2{Ni(dppe)}2]- yielded neutral, paramagnetic V(SNS)2{Ni(dppe)}2. The solid-state structure of V(SNS)2{Ni(dppe)}2 revealed that the two nickel ions occupy unique coordination environments: one nickel is in a square-planar S2P2 coordination environment (τ4 = 0.19), with a long Ni···V distance of 3.45 Å; the other nickel is in a tetrahedral S2P2 coordination environment (τ4 = 0.84) with a short Ni-V distance of 2.60 Å, consistent with a formal metal-metal bond. Continuous-wave X-band electron paramagnetic resonance spectroscopy, electrochemical investigations, and density functional theory computations indicated that the unpaired electron in the neutral V(SNS)2{Ni(dppe)}2 cluster is localized on the bridging [V(SNS)2] metalloligand, and as a result, V(SNS)2{Ni(dppe)}2 is best described as a two-electron mixed-valence complex. These results demonstrate the important role that metal-metal interactions and flexible coordination geometries play in enabling multiple, reversible electron transfer processes in small cluster complexes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Gamelin D. R.; Bominaar E. L.; Kirk M. L.; Wieghardt K.; Solomon E. I. Excited-State Contributions to Ground-State Properties of Mixed-Valence Dimers: Spectral and Electronic-Structural Studies of [Fe2(OH)3(tmtacn)2]2+ Related to the [Fe2S2]+ Active Sites of Plant-Type Ferredoxins. J. Am. Chem. Soc. 1996, 118, 8085–8097. 10.1021/ja9536633. - DOI
-
- Park J. G.; Collins B. A.; Darago L. E.; Runčevski T.; Ziebel M. E.; Aubrey M. L.; Jiang H. Z. H.; Velasquez E.; Green M. A.; Goodpaster J. D.; Long J. R. Magnetic Ordering through Itinerant Ferromagnetism in a Metal–Organic Framework. Nat. Chem. 2021, 13, 594–598. 10.1038/s41557-021-00666-6. - DOI - PubMed
-
- Zener C. Interaction between the d-Shells in the Transition Metals. II. Ferromagnetic Compounds of Manganese with Perovskite Structure. Phys. Rev. 1951, 82, 403–405. 10.1103/physrev.82.403. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

