Concepts of association between cancer and ionising radiation: accounting for specific biological mechanisms
- PMID: 36633666
- PMCID: PMC9950217
- DOI: 10.1007/s00411-022-01012-1
Concepts of association between cancer and ionising radiation: accounting for specific biological mechanisms
Abstract
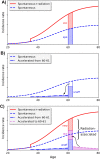
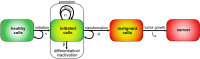
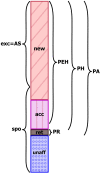
The probability that an observed cancer was caused by radiation exposure is usually estimated using cancer rates and risk models from radioepidemiological cohorts and is called assigned share (AS). This definition implicitly assumes that an ongoing carcinogenic process is unaffected by the studied radiation exposure. However, there is strong evidence that radiation can also accelerate an existing clonal development towards cancer. In this work, we define different association measures that an observed cancer was newly induced, accelerated, or retarded. The measures were quantified exemplarily by Monte Carlo simulations that track the development of individual cells. Three biologically based two-stage clonal expansion (TSCE) models were applied. In the first model, radiation initiates cancer development, while in the other two, radiation has a promoting effect, i.e. radiation accelerates the clonal expansion of pre-cancerous cells. The parameters of the TSCE models were derived from breast cancer data from the atomic bomb survivors of Hiroshima and Nagasaki. For exposure at age 30, all three models resulted in similar estimates of AS at age 60. For the initiation model, estimates of association were nearly identical to AS. However, for the promotion models, the cancerous clonal development was frequently accelerated towards younger ages, resulting in associations substantially higher than AS. This work shows that the association between a given cancer and exposure in an affected person depends on the underlying biological mechanism and can be substantially larger than the AS derived from classic radioepidemiology.
Keywords: Assigned share; Carcinogenesis; Probability of association; Radiation cancer risk; Two-stage clonal expansion model.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Biologically based analysis of lung cancer incidence in a large Canadian occupational cohort with low-dose ionizing radiation exposure, and comparison with Japanese atomic bomb survivors.J Toxicol Environ Health A. 2006 Jun;69(11):1013-38. doi: 10.1080/00397910500360202. J Toxicol Environ Health A. 2006. PMID: 16840251
-
Modeling of cell inactivation and carcinogenesis in the atomic bomb survivors with applications to the mortality from all solid, stomach and liver cancer.Radiat Environ Biophys. 2008 Jul;47(3):375-88. doi: 10.1007/s00411-008-0169-9. Epub 2008 May 15. Radiat Environ Biophys. 2008. PMID: 18481074
-
Modelling of carcinogenesis and low-dose hypersensitivity: an application to lung cancer incidence among atomic bomb survivors.Radiat Environ Biophys. 2004 Feb;42(4):265-73. doi: 10.1007/s00411-003-0215-6. Epub 2003 Dec 16. Radiat Environ Biophys. 2004. PMID: 14676961
-
Cancer and non-cancer effects in Japanese atomic bomb survivors.J Radiol Prot. 2009 Jun;29(2A):A43-59. doi: 10.1088/0952-4746/29/2A/S04. Epub 2009 May 19. J Radiol Prot. 2009. PMID: 19454804 Review.
-
The use of biologically based cancer risk models in radiation epidemiology.Radiat Prot Dosimetry. 2003;104(4):367-76. doi: 10.1093/oxfordjournals.rpd.a006200. Radiat Prot Dosimetry. 2003. PMID: 14579893 Review.
Cited by
-
Minimum latency effects for cancer associated with exposures to radiation or other carcinogens.Br J Cancer. 2024 Mar;130(5):819-829. doi: 10.1038/s41416-023-02544-z. Epub 2024 Jan 11. Br J Cancer. 2024. PMID: 38212483 Free PMC article.
References
-
- Alexandrov LB, Kim J, Haradhvala NJ, Huang MN, Tian Ng AW, Wu Y, Boot A, Covington KR, Gordenin DA, Bergstrom EN, Islam SMA, Lopez-Bigas N, Klimczak LJ, McPherson JR, Morganella S, Sabarinathan R, Wheeler DA, Mustonen V, PCAWG Mutational Signatures Working Group. Getz G, Rozen SG, Stratton MR, PCAWG Consortium The repertoire of mutational signatures in human cancer. Nature. 2020;578(7793):94–101. doi: 10.1038/s41586-020-1943-3. - DOI - PMC - PubMed
-
- Behjati S, Gundem G, Wedge DC, Roberts ND, Tarpey PS, Cooke SL, Van Loo P, Alexandrov LB, Ramakrishna M, Davies H, Nik-Zainal S, Hardy C, Latimer C, Raine KM, Stebbings L, Menzies A, Jones D, Shepherd R, Butler AP, Teague JW, Jorgensen M, Khatri B, Pillay N, Shlien A, Futreal PA, Badie C, ICGC Prostate Group. McDermott U, Bova GS, Richardson AL, Flanagan AM, Stratton MR, Campbell PJ. Mutational signatures of ionizing radiation in second malignancies. Nat Commun. 2016;7:12605. doi: 10.1038/ncomms12605. - DOI - PMC - PubMed
-
- Bernstein JL, Thomas DC, Shore RE, Robson M, Boice JD, Jr, Stovall M, Andersson M, Bernstein L, Malone KE, Reiner AS, et al. Contralateral breast cancer after radiotherapy among BRCA1 and BRCA2 mutation carriers: a WECARE study report. Eur J Cancer. 2013;49(14):2979–2985. doi: 10.1016/j.ejca.2013.04.028. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

