Population Pharmacokinetics of Piperacillin/Tazobactam Across the Adult Lifespan
- PMID: 36633812
- PMCID: PMC9969806
- DOI: 10.1007/s40262-022-01198-z
Population Pharmacokinetics of Piperacillin/Tazobactam Across the Adult Lifespan
Abstract
Background and objective: Piperacillin/tazobactam is one of the most frequently used antimicrobials in older adults. Using an opportunistic study design, we evaluated the pharmacokinetics of piperacillin/tazobactam as a probe drug to evaluate changes in antibacterial drug exposure and dosing requirements, including in older adults.
Methods: A total of 121 adult patients were included. The population pharmacokinetic models that best characterized the observed plasma concentrations of piperacillin and tazobactam were one-compartment structural models with zero-order input and linear elimination.
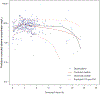
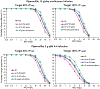
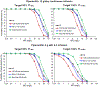
Results: Among all potential covariates, estimated creatinine clearance had the most substantial impact on the elimination clearance for both piperacillin and tazobactam. After accounting for renal function and body size, there was no remaining impact of frailty on the pharmacokinetics of piperacillin and tazobactam. Monte Carlo simulations indicated that renal function had a greater impact on the therapeutic target attainment than age, although these covariates were highly correlated. Frailty, using the Canadian Study of Health and Aging Clinical Frailty Scale, was assessed in 60 patients who were ≥ 65 years of age.
Conclusions: The simulations suggested that adults ≤ 50 years of age infected with organisms with higher minimum inhibitory concentrations may benefit from continuous piperacillin/tazobactam infusions (12 g/day of piperacillin component) or extended infusions of 4 g every 8 hours. However, for a target of 50% fT + minimum inhibitory concentration, dosing based on renal function is generally preferable to dosing by age, and simulations suggested that patients with creatinine clearance ≥ 120 mL/min may benefit from infusions of 4 g every 8 hours for organisms with higher minimum inhibitory concentrations.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Conflicts of Interest
SJB receives support from the National Institutes of Health, the US Food and Drug Administration, the Patient-Centered Outcomes Research Institute, the Rheumatology Research Foundation’s Scientist Development Award, the Childhood Arthritis and Rheumatology Research Alliance, Purdue Pharma, and consulting for UCB.
Figures

References
-
- Cusack BJ. 2004. Pharmacokinetics in older persons. Am J Geriatr Pharmacother. 2004;2:274–302. - PubMed
-
- Rule AD, Gussak HM, Pond GR, et al. Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis. 2004;43:112–19. - PubMed
-
- Benson JM. Antimicrobial pharmacokinetics and pharmacodynamics in older adults. Infect Dis Clin North Am. 2017;31:609–17. - PubMed
-
- United States Food and Drug Administration (FDA). Piperacillin/tazobactam for injection, Wyeth Pharmaceuticals, Philadelphia, PA. FDA; web site. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/050684s88s89s9.... Published 2015. Updated May 2017. Accessed July 11, 2022.
Publication types
MeSH terms
Substances
Grants and funding
- HHSN272201300020I/AI/NIAID NIH HHS/United States
- K24 AI143971/AI/NIAID NIH HHS/United States
- HHSN275201000003C/HD/NICHD NIH HHS/United States
- HHSN272201300021C/AI/NIAID NIH HHS/United States
- HHSN275201000003I/HD/NICHD NIH HHS/United States
- HHSN272201500002C/AI/NIAID NIH HHS/United States
- P30 AG028716/AG/NIA NIH HHS/United States
- T32 AI100851/AI/NIAID NIH HHS/United States
- HHSN272201300017C/AI/NIAID NIH HHS/United States
- HHSN272201300017I/AI/NIAID NIH HHS/United States
- U24 MD016258/MD/NIMHD NIH HHS/United States
- K23 AR075874/AR/NIAMS NIH HHS/United States
- 1U24-MD016258/NH/NIH HHS/United States
- HHSN272201500006C/AI/NIAID NIH HHS/United States
- U18 FD006298/FD/FDA HHS/United States
LinkOut - more resources
Full Text Sources

