Effect of Fluvoxamine vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial
- PMID: 36633838
- PMCID: PMC9857647
- DOI: 10.1001/jama.2022.24100
Effect of Fluvoxamine vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial
Erratum in
-
Revisions to Medication Description and Manufacturer.JAMA. 2023 Oct 24;330(16):1589. doi: 10.1001/jama.2023.20908. JAMA. 2023. PMID: 37874583 Free PMC article. No abstract available.
-
Revisions to Role of Funder/Sponsor Statement.JAMA. 2024 Sep 11;332(14):1211. doi: 10.1001/jama.2024.18425. Online ahead of print. JAMA. 2024. PMID: 39259565 Free PMC article. No abstract available.
Abstract
Importance: The effectiveness of fluvoxamine to shorten symptom duration or prevent hospitalization among outpatients with mild to moderate symptomatic COVID-19 is unclear.
Objective: To evaluate the efficacy of low-dose fluvoxamine (50 mg twice daily) for 10 days compared with placebo for the treatment of mild to moderate COVID-19 in the US.
Design, setting, and participants: The ongoing Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) platform randomized clinical trial was designed to test repurposed medications in outpatients with mild to moderate COVID-19. A total of 1288 participants aged 30 years or older with test-confirmed SARS-CoV-2 infection and experiencing 2 or more symptoms of acute COVID-19 for 7 days or less were enrolled between August 6, 2021, and May 27, 2022, at 91 sites in the US.
Interventions: Participants were randomized to receive 50 mg of fluvoxamine twice daily for 10 days or placebo.
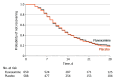
Main outcomes and measures: The primary outcome was time to sustained recovery (defined as the third day of 3 consecutive days without symptoms). There were 7 secondary outcomes, including a composite outcome of hospitalization, urgent care visit, emergency department visit, or death through day 28.
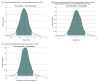
Results: Among 1331 participants who were randomized (median age, 47 years [IQR, 38-57 years]; 57% were women; and 67% reported receiving ≥2 doses of a SARS-CoV-2 vaccine), 1288 completed the trial (674 in the fluvoxamine group and 614 in the placebo group). The median time to sustained recovery was 12 days (IQR, 11-14 days) in the fluvoxamine group and 13 days (IQR, 12-13 days) in the placebo group (hazard ratio [HR], 0.96 [95% credible interval, 0.86-1.06], posterior P = .21 for the probability of benefit [determined by an HR >1]). For the composite outcome, 26 participants (3.9%) in the fluvoxamine group were hospitalized, had an urgent care visit, had an emergency department visit, or died compared with 23 participants (3.8%) in the placebo group (HR, 1.1 [95% credible interval, 0.5-1.8], posterior P = .35 for the probability of benefit [determined by an HR <1]). One participant in the fluvoxamine group and 2 participants in the placebo group were hospitalized; no deaths occurred in either group. Adverse events were uncommon in both groups.
Conclusions and relevance: Among outpatients with mild to moderate COVID-19, treatment with 50 mg of fluvoxamine twice daily for 10 days, compared with placebo, did not improve time to sustained recovery. These findings do not support the use of fluvoxamine at this dose and duration in patients with mild to moderate COVID-19.
Trial registration: ClinicalTrials.gov Identifier: NCT04885530.
Conflict of interest statement
Figures
Comment in
-
Lack of Benefit of Fluvoxamine for COVID-19.JAMA. 2023 Jan 24;329(4):291-292. doi: 10.1001/jama.2022.23954. JAMA. 2023. PMID: 36633871 No abstract available.
-
In outpatients with mild to moderate COVID-19, low-dose fluvoxamine did not reduce time to sustained recovery.Ann Intern Med. 2023 May;176(5):JC52. doi: 10.7326/J23-0025. Epub 2023 May 2. Ann Intern Med. 2023. PMID: 37126816
-
Fluvoxamine vs Placebo and Time to Recovery in Outpatients With Mild to Moderate COVID-19.JAMA. 2023 May 16;329(19):1702. doi: 10.1001/jama.2023.5061. JAMA. 2023. PMID: 37191708 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

