COVID-19 Convalescent Plasma for the Treatment of Immunocompromised Patients: A Systematic Review and Meta-analysis
- PMID: 36633846
- PMCID: PMC9857047
- DOI: 10.1001/jamanetworkopen.2022.50647
COVID-19 Convalescent Plasma for the Treatment of Immunocompromised Patients: A Systematic Review and Meta-analysis
Abstract
Importance: Patients who are immunocompromised have increased risk for morbidity and mortality associated with coronavirus disease 2019 (COVID-19) because they less frequently mount antibody responses to vaccines. Although neutralizing anti-spike monoclonal-antibody treatment has been widely used to treat COVID-19, evolutions of SARS-CoV-2 have been associated with monoclonal antibody-resistant SARS-CoV-2 variants and greater virulence and transmissibility of SARS-CoV-2. Thus, the therapeutic use of COVID-19 convalescent plasma has increased on the presumption that such plasma contains potentially therapeutic antibodies to SARS-CoV-2 that can be passively transferred to the plasma recipient.
Objective: To assess the growing number of reports of clinical experiences of patients with COVID-19 who are immunocompromised and treated with specific neutralizing antibodies via COVID-19 convalescent plasma transfusion.
Data sources: On August 12, 2022, a systematic search was performed for clinical studies of COVID-19 convalescent plasma use in patients who are immunocompromised.
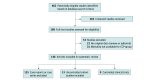
Study selection: Randomized clinical trials, matched cohort studies, and case report or series on COVID-19 convalescent plasma use in patients who are immunocompromised were included. The electronic search yielded 462 unique records, of which 199 were considered for full-text screening.
Data extraction and synthesis: The study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Data were extracted by 3 independent reviewers in duplicate and pooled.
Main outcomes and meaures: The prespecified end point was all-cause mortality after COVID-19 convalescent plasma transfusion; exploratory subgroup analyses were performed based on putative factors associated with the potential mortality benefit of convalescent plasma.
Results: This systematic review and meta-analysis included 3 randomized clinical trials enrolling 1487 participants and 5 controlled studies. Additionally, 125 case series or reports enrolling 265 participants and 13 uncontrolled large case series enrolling 358 participants were included. Separate meta-analyses, using models both stratified and pooled by study type (ie, randomized clinical trials and matched cohort studies), demonstrated that transfusion of COVID-19 convalescent plasma was associated with a decrease in mortality compared with the control cohort for the amalgam of both randomized clinical trials and matched cohort studies (risk ratio [RR], 0.63 [95% CI, 0.50-0.79]).
Conclusions and relevance: These findings suggest that transfusion of COVID-19 convalescent plasma is associated with mortality benefit for patients who are immunocompromised and have COVID-19.
Conflict of interest statement
Figures

References
-
- World Health Organization (WHO) . Coronavirus disease (COVID-19). Accessed July 10, 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
-
- WHO . Therapeutics and COVID-19: living guideline. Accessed August 1, 2022. https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.4 - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

