Lactate as a myokine and exerkine: drivers and signals of physiology and metabolism
- PMID: 36633863
- PMCID: PMC9970662
- DOI: 10.1152/japplphysiol.00497.2022
Lactate as a myokine and exerkine: drivers and signals of physiology and metabolism
Abstract
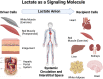
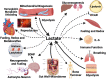
No longer viewed as a metabolic waste product and cause of muscle fatigue, a contemporary view incorporates the roles of lactate in metabolism, sensing and signaling in normal as well as pathophysiological conditions. Lactate exists in millimolar concentrations in muscle, blood, and other tissues and can rise more than an order of magnitude as the result of increased production and clearance limitations. Lactate exerts its powerful driver-like influence by mass action, redox change, allosteric binding, and other mechanisms described in this article. Depending on the condition, such as during rest and exercise, following carbohydrate nutrition, injury, or pathology, lactate can serve as a myokine or exerkine with autocrine-, paracrine-, and endocrine-like functions that have important basic and translational implications. For instance, lactate signaling is: involved in reproductive biology, fueling the heart, muscle adaptation, and brain executive function, growth and development, and a treatment for inflammatory conditions. Lactate also works with many other mechanisms and factors in controlling cardiac output and pulmonary ventilation during exercise. Ironically, lactate can be disruptive of normal processes such as insulin secretion when insertion of lactate transporters into pancreatic β-cell membranes is not suppressed, and in carcinogenesis when factors that suppress carcinogenesis are inhibited, whereas factors that promote carcinogenesis are upregulated. Lactate signaling is important in areas of intermediary metabolism, redox biology, mitochondrial biogenesis, neurobiology, gut physiology, appetite regulation, nutrition, and overall health and vigor. The various roles of lactate as a myokine and exerkine are reviewed.NEW & NOTEWORTHY Lactate sensing and signaling is a relatively new and rapidly changing field. As a physiological signal lactate works both independently and in concert with other signals. Lactate operates via covalent binding and canonical signaling, redox change, and lactylation of DNA. Lactate can also serve as an element of feedback loops in cardiopulmonary regulation. From conception through aging lactate is not the only a myokine or exerkine, but it certainly deserves consideration as a physiological signal.
Keywords: cardiopulmonary regulation; glucose paradox; lactate shuttle; lactylation; metabolic signaling.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Brooks GA. Lactate: glycolytic end product and oxidative substrate during sustained exercise in mammals—the “lactate shuttle”. In: Circulation, Respiration, and Metabolism. Proceedings in Life Sciences, edited by Gilles R. Berlin, Heidelberg: Springer, 1985, p. 208–218.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

