Magnetic Resonance Imaging-Guided vs Computed Tomography-Guided Stereotactic Body Radiotherapy for Prostate Cancer: The MIRAGE Randomized Clinical Trial
- PMID: 36633877
- PMCID: PMC9857817
- DOI: 10.1001/jamaoncol.2022.6558
Magnetic Resonance Imaging-Guided vs Computed Tomography-Guided Stereotactic Body Radiotherapy for Prostate Cancer: The MIRAGE Randomized Clinical Trial
Abstract
Importance: Magnetic resonance imaging (MRI) guidance offers multiple theoretical advantages in the context of stereotactic body radiotherapy (SBRT) for prostate cancer. However, to our knowledge, these advantages have yet to be demonstrated in a randomized clinical trial.
Objective: To determine whether aggressive margin reduction with MRI guidance significantly reduces acute grade 2 or greater genitourinary (GU) toxic effects after prostate SBRT compared with computed tomography (CT) guidance.
Design, setting, and participants: This phase 3 randomized clinical trial (MRI-Guided Stereotactic Body Radiotherapy for Prostate Cancer [MIRAGE]) enrolled men aged 18 years or older who were receiving SBRT for clinically localized prostate adenocarcinoma at a single center between May 5, 2020, and October 1, 2021. Data were analyzed from January 15, 2021, through May 15, 2022. All patients had 3 months or more of follow-up.
Interventions: Patients were randomized 1:1 to SBRT with CT guidance (control arm) or MRI guidance. Planning margins of 4 mm (CT arm) and 2 mm (MRI arm) were used to deliver 40 Gy in 5 fractions.
Main outcomes and measures: The primary end point was the incidence of acute (≤90 days after SBRT) grade 2 or greater GU toxic effects (using Common Terminology Criteria for Adverse Events, version 4.03 [CTCAE v4.03]). Secondary outcomes included CTCAE v4.03-based gastrointestinal toxic effects and International Prostate Symptom Score (IPSS)-based and Expanded Prostate Cancer Index Composite-26 (EPIC-26)-based outcomes.
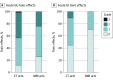
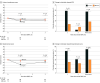
Results: Between May 2020 and October 2021, 156 patients were randomized: 77 to CT (median age, 71 years [IQR, 67-77 years]) and 79 to MRI (median age, 71 years [IQR, 68-75 years]). A prespecified interim futility analysis conducted after 100 patients reached 90 or more days after SBRT was performed October 1, 2021, with the sample size reestimated to 154 patients. Thus, the trial was closed to accrual early. The incidence of acute grade 2 or greater GU toxic effects was significantly lower with MRI vs CT guidance (24.4% [95% CI, 15.4%-35.4%] vs 43.4% [95% CI, 32.1%-55.3%]; P = .01), as was the incidence of acute grade 2 or greater gastrointestinal toxic effects (0.0% [95% CI, 0.0%-4.6%] vs 10.5% [95% CI, 4.7%-19.7%]; P = .003). Magnetic resonance imaging guidance was associated with a significantly smaller percentage of patients with a 15-point or greater increase in IPSS at 1 month (6.8% [5 of 72] vs 19.4% [14 of 74]; P = .01) and a significantly reduced percentage of patients with a clinically significant (≥12-point) decrease in EPIC-26 bowel scores (25.0% [17 of 68] vs 50.0% [34 of 68]; P = .001) at 1 month.
Conclusions and relevance: In this randomized clinical trial, compared with CT-guidance, MRI-guided SBRT significantly reduced both moderate acute physician-scored toxic effects and decrements in patient-reported quality of life. Longer-term follow-up will confirm whether these notable benefits persist.
Trial registration: ClinicalTrials.gov Identifier: NCT04384770.
Conflict of interest statement
Figures

Comment in
-
The MIRAGE Trial-Optical Illusion or the Future of Prostate Stereotactic Radiotherapy?JAMA Oncol. 2023 Mar 1;9(3):373-375. doi: 10.1001/jamaoncol.2022.6334. JAMA Oncol. 2023. PMID: 36633862 Clinical Trial. No abstract available.
-
Magnetic resonance imaging-guided stereotactic body radiotherapy for prostate cancer: more than a simple "MIRAGE"?Transl Cancer Res. 2023 Oct 31;12(10):2454-2457. doi: 10.21037/tcr-23-611. Epub 2023 Oct 7. Transl Cancer Res. 2023. PMID: 37969364 Free PMC article. No abstract available.
References
-
- Brand DH, Tree AC, Ostler P, et al. ; PACE Trial Investigators . Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019;20(11):1531-1543. doi:10.1016/S1470-2045(19)30569-8 - DOI - PMC - PubMed

