Metagenomics Shines Light on the Evolution of "Sunscreen" Pigment Metabolism in the Teloschistales (Lichen-Forming Ascomycota)
- PMID: 36634008
- PMCID: PMC9907504
- DOI: 10.1093/gbe/evad002
Metagenomics Shines Light on the Evolution of "Sunscreen" Pigment Metabolism in the Teloschistales (Lichen-Forming Ascomycota)
Abstract
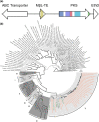
Fungi produce a vast number of secondary metabolites that shape their interactions with other organisms and the environment. Characterizing the genes underpinning metabolite synthesis is therefore key to understanding fungal evolution and adaptation. Lichenized fungi represent almost one-third of Ascomycota diversity and boast impressive secondary metabolites repertoires. However, most lichen biosynthetic genes have not been linked to their metabolite products. Here we used metagenomic sequencing to survey gene families associated with production of anthraquinones, UV-protectant secondary metabolites present in various fungi, but especially abundant in a diverse order of lichens, the Teloschistales (class Lecanoromycetes, phylum Ascomycota). We successfully assembled 24 new, high-quality lichenized-fungal genomes de novo and combined them with publicly available Lecanoromycetes genomes from taxa with diverse secondary chemistry to produce a whole-genome tree. Secondary metabolite biosynthetic gene cluster (BGC) analysis showed that whilst lichen BGCs are numerous and highly dissimilar, core enzyme genes are generally conserved across taxa. This suggests metabolite diversification occurs via re-shuffling existing enzyme genes with novel accessory genes rather than BGC gains/losses or de novo gene evolution. We identified putative anthraquinone BGCs in our lichen dataset that appear homologous to anthraquinone clusters from non-lichenized fungi, suggesting these genes were present in the common ancestor of the subphylum Pezizomycotina. Finally, we identified unique transporter genes in Teloschistales anthraquinone BGCs that may explain why these metabolites are so abundant and ubiquitous in these lichens. Our results support the importance of metagenomics for understanding the secondary metabolism of non-model fungi such as lichens.
Keywords: Lecanoromycetes; ABC-transporter; anthraquinone; biosynthetic gene cluster; fungal evolution; lichenized fungi.
© The Author(s) 2023. Published by Oxford University Press on behalf of Society for Molecular Biology and Evolution.
Figures



References
-
- Alneberg J, et al. . 2014. Binning metagenomic contigs by coverage and composition. Nat Methods. 11:1144–1146. - PubMed
-
- Amnuaykanjanasin A, Daub ME. 2009. The ABC transporter ATR1 is necessary for efflux of the toxin cercosporin in the fungus Cercospora nicotianae. Fungal Genet Biol. 46:146–158. - PubMed
-
- Andrade AC, Del Sorbo G, Van Nistelrooy JGM, De WM. 2000. The ABC transporter AtrB from Aspergillus nidulans mediates resistance to all major classes of fungicides and some natural toxic compounds. Microbiology 146:1987–1997. - PubMed
-
- Andrews S, Krueger F, Seconds-Pichon A, Biggins F, Wingett S. 2015. FastQC. A quality control tool for high throughput sequence data. Babraham Bioinform. 1:1.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

