Vaccination Coverage by Age 24 Months Among Children Born During 2018-2019 - National Immunization Survey-Child, United States, 2019-2021
- PMID: 36634013
- PMCID: PMC9869730
- DOI: 10.15585/mmwr.mm7202a3
Vaccination Coverage by Age 24 Months Among Children Born During 2018-2019 - National Immunization Survey-Child, United States, 2019-2021
Abstract
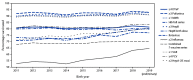
Millions of young children are vaccinated safely in the United States each year against a variety of potentially dangerous infectious diseases (1). The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination against 14 diseases during the first 24 months of life* (2). This report describes vaccination coverage by age 24 months using data from the National Immunization Survey-Child (NIS-Child).† Compared with coverage among children born during 2016-2017, coverage among children born during 2018-2019 increased for a majority of recommended vaccines. Coverage was >90% for ≥3 doses of poliovirus vaccine (93.4%), ≥3 doses of hepatitis B vaccine (HepB) (92.7%), ≥1 dose of measles, mumps, and rubella vaccine (MMR) (91.6%), and ≥1 dose of varicella vaccine (VAR) (91.1%); coverage was lowest for ≥2 doses of hepatitis A vaccine (HepA) (47.3%). Vaccination coverage overall was similar or higher among children reaching age 24 months during March 2020 or later (during the COVID-19 pandemic) than among those reaching age 24 months before March 2020 (prepandemic); however, coverage with the combined 7-vaccine series§ among children living below the federal poverty level or in rural areas decreased by 4-5 percentage points during the pandemic (3). Among children born during 2018-2019, coverage disparities were observed by race and ethnicity, poverty status, health insurance status, and Metropolitan Statistical Area (MSA) residence. Coverage was typically higher among privately insured children than among children with other insurance or no insurance. Persistent disparities by health insurance status indicate the need to improve access to vaccines through the Vaccines for Children (VFC) program.¶ Providers should review children's histories and recommend needed vaccinations during every clinical encounter and address parental hesitancy to help reduce disparities and ensure that all children are protected from vaccine-preventable diseases.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
-
- Wodi AP, Ault K, Hunter P, McNally V, Szilagyi PG, Bernstein H. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2021. MMWR Morb Mortal Wkly Rep 2021;70:189–92. 10.15585/mmwr.mm7006a1 - DOI - PMC - PubMed
-
- CDC. Childhood vaccination coverage before and during the COVID-19 pandemic among children born January 2017–May 2020, National Immunization Survey-Child (NIS-Child), 2018–2021. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. https://www.cdc.gov/vaccines/imz-managers/coverage/childvaxview/pubs-pre...
-
- DeSilva MB, Haapala J, Vazquez-Benitez G, et al. Association of the COVID-19 pandemic with routine childhood vaccination rates and proportion up to date with vaccinations across 8 US health systems in the Vaccine Safety Datalink. JAMA Pediatr 2022;176:68–77. 10.1001/jamapediatrics.2021.4251 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

