Fra-1 induces apoptosis and neuroinflammation by targeting S100A8 to modulate TLR4 pathways in spinal cord ischemia/reperfusion injury
- PMID: 36634215
- PMCID: PMC9836372
- DOI: 10.1111/bpa.13113
Fra-1 induces apoptosis and neuroinflammation by targeting S100A8 to modulate TLR4 pathways in spinal cord ischemia/reperfusion injury
Abstract
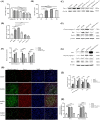
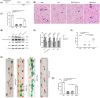
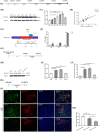
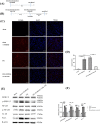
Spinal cord ischemia/reperfusion injury (SCII) is a severe complication driven by apoptosis and neuroinflammation. An increase in the expression of c-Fos, a member of the AP-1 family, is known as a neuronal activation marker in SCII. The AP-1 family is composed of Jun, Fos, and is associated with the regulation of cytokines expression and apoptosis. Fra-1 is a member of the Fos family, however, the contribution of Fra-1 to SCII is still unclear. In our study, Fra-1 was highly upregulated especially in neurons and microglia and promoted apoptosis by changing the expression of Bax/Bcl-2 after SCII. Furthermore, we found that Fra-1 directly regulated the transcription expression of S100A8. We demonstrated that knockdown of Fra-1 alleviated S100A8 mediated neuronal apoptosis and inflammatory factor release, thus improved motor function after SCII. Interestingly, we showed that administration of TAK-242, the TLR4 inhibitor, to the ischemia/reperfusion (I/R) injury induced rats suppressed the activation of the ERK and NF-κB pathways, and further reduced Fra-1 expression. In conclusion, we found that Fra-1-targeted S100A8 was expressed the upstream of Fra-1, and the Fra-1/S100A8 interaction formed a feedback loop in the signaling pathways activated by SCII.
Keywords: apoptosis; feedback loop; ischemia/reperfusion; neuroinflammation; oxygen-glucose deprivation/reoxygenation; spinal cord ischemia/reperfusion injury.
© 2022 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Inhibition of heat shock protein family A member 8 attenuates spinal cord ischemia-reperfusion injury via astrocyte NF-κB/NLRP3 inflammasome pathway : HSPA8 inhibition protects spinal ischemia-reperfusion injury.J Neuroinflammation. 2021 Aug 6;18(1):170. doi: 10.1186/s12974-021-02220-0. J Neuroinflammation. 2021. PMID: 34362408 Free PMC article.
-
Administration with curcumin alleviates spinal cord ischemia-reperfusion injury by regulating anti-oxidative stress and microglia activation-mediated neuroinflammation via Nrf2/NF-κB axis.In Vitro Cell Dev Biol Anim. 2024 Mar;60(2):172-182. doi: 10.1007/s11626-023-00846-3. Epub 2024 Jan 16. In Vitro Cell Dev Biol Anim. 2024. PMID: 38228998
-
Elevated microRNA-214-3p level ameliorates neuroinflammation after spinal cord ischemia-reperfusion injury by inhibiting Nmb/Cav3.2 pathway.Int Immunopharmacol. 2024 May 30;133:112031. doi: 10.1016/j.intimp.2024.112031. Epub 2024 Apr 16. Int Immunopharmacol. 2024. PMID: 38631219
-
USP18 overexpression protects against spinal cord ischemia/reperfusion injury via regulating autophagy.Neurosci Lett. 2023 Jul 27;810:137359. doi: 10.1016/j.neulet.2023.137359. Epub 2023 Jun 24. Neurosci Lett. 2023. PMID: 37356565
-
Neuroprotective Effects of Luteolin Against Spinal Cord Ischemia-Reperfusion Injury by Attenuation of Oxidative Stress, Inflammation, and Apoptosis.J Med Food. 2018 Jan;21(1):13-20. doi: 10.1089/jmf.2017.4021. Epub 2017 Oct 4. J Med Food. 2018. PMID: 28976796
Cited by
-
Tungsten-based polyoxometalate nanoclusters as ferroptosis inhibitors modulating S100A8/A9-mediated iron metabolism pathway for managing intracerebral haemorrhage.J Nanobiotechnology. 2025 Feb 19;23(1):122. doi: 10.1186/s12951-025-03149-9. J Nanobiotechnology. 2025. PMID: 39972331 Free PMC article.
-
Blockade of the ADAM8-Fra-1 complex attenuates neuroinflammation by suppressing the Map3k4/MAPKs axis after spinal cord injury.Cell Mol Biol Lett. 2024 May 16;29(1):75. doi: 10.1186/s11658-024-00589-3. Cell Mol Biol Lett. 2024. PMID: 38755530 Free PMC article.
-
Time varying characteristic in somatosensory evoked potentials as a biomarker of spinal cord ischemic-reperfusion injury in rat.Front Neurosci. 2024 Sep 9;18:1411016. doi: 10.3389/fnins.2024.1411016. eCollection 2024. Front Neurosci. 2024. PMID: 39315075 Free PMC article.
-
Steroid inhibited Serpina3n expression which was positively correlated with the degrees of spinal cord injury.Heliyon. 2024 Feb 29;10(5):e26649. doi: 10.1016/j.heliyon.2024.e26649. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38449654 Free PMC article.
-
TAK-242, a toll-like receptor 4 antagonist, against brain injury by alleviates autophagy and inflammation in rats.Open Life Sci. 2023 Jul 29;18(1):20220662. doi: 10.1515/biol-2022-0662. eCollection 2023. Open Life Sci. 2023. PMID: 37528888 Free PMC article.
References
-
- Mi J, Yang Y, Yao H, Huan Z, Xu C, Ren Z, et al. Inhibition of heat shock protein family A member 8 attenuates spinal cord ischemia‐reperfusion injury via astrocyte NF‐kappaB/NLRP3 inflammasome pathway: HSPA8 inhibition protects spinal ischemia‐reperfusion injury. J Neuroinflammation. 2021;18:170. - PMC - PubMed
-
- Miranda V, Sousa J, Mansilha A. Spinal cord injury in endovascular thoracoabdominal aortic aneurysm repair: prevalence, risk factors and preventive strategies. Int Angiol. 2018;37:112–26. - PubMed
-
- Huang CK, Dai D, Xie H, Zhu Z, Hu J, Su M, et al. Lgr4 governs a pro‐inflammatory program in macrophages to antagonize post‐infarction cardiac repair. Circ Res. 2020;127:953–73. - PubMed
-
- Na J, Bak DH, Im SI, Choi H, Hwang JH, Kong SY, et al. Antiapoptotic effects of glycosaminoglycans via inhibition of ERK/AP1 signaling in TNFalphastimulated human dermal fibroblasts. Int J Mol Med. 2018;41:3090–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

