Efficacy of nirsevimab against respiratory syncytial virus lower respiratory tract infections in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital heart disease and chronic lung disease: a pooled analysis of randomised controlled trials
- PMID: 36634694
- PMCID: PMC9940918
- DOI: 10.1016/S2352-4642(22)00321-2
Efficacy of nirsevimab against respiratory syncytial virus lower respiratory tract infections in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital heart disease and chronic lung disease: a pooled analysis of randomised controlled trials
Abstract
Background: In a phase 2b trial and the phase 3 MELODY trial, nirsevimab, an extended half-life, monoclonal antibody against respiratory syncytial virus (RSV), protected healthy infants born preterm or at full term against medically attended RSV lower respiratory tract infection (LRTI). In the MEDLEY phase 2-3 trial in infants at higher risk for severe RSV infection, nirsevimab showed a similar safety profile to that of palivizumab. The aim of the current analysis was to assess the efficacy of nirsevimab using a weight-banded dosing regimen in infants born between 29 weeks gestational age and full term.
Methods: Infants enrolled in the phase 2b and MELODY trials were randomised (2:1) to receive a single intramuscular injection of nirsevimab (infants weighing <5 kg received 50 mg; those weighing ≥5 kg received 100 mg) or placebo before the RSV season. Infants in MEDLEY were randomised (2:1) to receive one dose of nirsevimab (infants weighing <5 kg received 50 mg; those weighing ≥5 kg received 100 mg) followed by four monthly placebo doses, or five once-a-month intramuscular doses of palivizumab. We report a prespecified pooled efficacy analysis assessing the weight-banded dosing regimen proposed on the basis of the phase 2b and MELODY trials, in addition to extrapolated efficacy in infants with chronic lung disease, congenital heart disease, or extreme preterm birth (<29 weeks' gestational age) based on pharmacokinetic data from the phase 2-3 MEDLEY safety trial. For the pooled efficacy analysis, the primary endpoint was incidence of medically attended RSV LRTI through 150 days post-dose. The secondary efficacy endpoint was number of admissions to hospital for medically attended RSV LRTI. The incidence of very severe RSV LRTI was an exploratory endpoint, defined as cases of hospital admission for medically attended RSV LRTI that required supplemental oxygen or intravenous fluids. We also did a prespecified exploratory analysis of medically attended LRTI of any cause (in the investigator's judgement) and hospital admission for respiratory illness of any cause (defined as any upper respiratory tract infection or LRTI leading to hospital admission). Post hoc exploratory analyses of outpatient visits and antibiotic use were also done. Nirsevimab serum concentrations in MEDLEY were assessed using population pharmacokinetic methods and the pooled data from the phase 2b and MELODY trials. An exposure target was defined on the basis of an exposure-response analysis. To successfully demonstrate extrapolation, more than 80% of infants in MEDLEY had to achieve serum nirsevimab exposures at or above the predicted efficacious target.
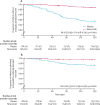
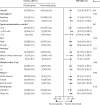
Findings: Overall, 2350 infants (1564 in the nirsevimab group and 786 in the placebo group) in the phase 2b and MELODY trials were included in the pooled analysis. Nirsevimab showed efficacy versus placebo with respect to the primary endpoint of medically attended RSV LRTI (19 [1%] nirsevimab recipients vs 51 [6%] placebo recipients; relative risk reduction [RRR] 79·5% [95% CI 65·9-87·7]). Consistent efficacy was shown for additional endpoints of RSV LRTI hospital admission (nine [1%] nirsevimab recipients vs 21 [3%] placebo recipients; 77·3% [50·3-89·7]) and very severe RSV (five [<1%] vs 18 [2%]; 86·0% [62·5-94·8]). Nirsevimab recipients had fewer hospital admissions for any-cause respiratory illness (RRR 43·8% [18·8-61·1]), any-cause medically attended LRTI (35·4% [21·5-46·9]), LRTI outpatient visits (41·9% [25·7-54·6]), and antibiotic prescriptions (23·6% [3·8-39·3]). Among infants with chronic lung disease, congenital heart disease, or extreme preterm birth in MEDLEY, nirsevimab serum exposures were similar to those found in the pooled data; exposures were above the target in more than 80% of the overall MEDLEY trial population (94%), including infants with chronic lung disease (94%) or congenital heart disease (80%) and those born extremely preterm (94%).
Interpretation: A single dose of nirsevimab protected healthy infants born at term or preterm from medically attended RSV LRTI, associated hospital admission, and severe RSV. Pharmacokinetic data support efficacy extrapolation to infants with chronic lung disease, congenital heart disease, or extreme prematurity. Together, these data suggest that nirsevimab has the potential to change the landscape of infant RSV disease by reducing a major cause of infant morbidity and the consequent burden on caregivers, clinicians, and health-care providers.
Funding: AstraZeneca and Sanofi.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests EAFS has received grants or contracts from AstraZeneca, Johnson and Johnson, Merck, Pfizer, and Roche; consulting fees from Adiago Therapeutics, Cidara Therapeutics, Merck, Nuance Pharmaceuticals, Pfizer, and Sanofi; payment or honoraria from AstraZeneca and Pfizer; support for meeting attendance and/or travel from AstraZeneca; and has participated in data safety monitoring boards or advisory boards for Abbvie, the Bill and Melinda Gates Foundation, and GSK. SAM has received grants or contracts from the Bill and Melinda Gates Foundation, GSK, Minervax, Pfizer, and the South African Medical Research Council; payments or honoraria from the Bill and Melinda Gates Foundation; and has participated in data safety monitoring boards or advisory boards for PATH and CAPRISA. WJM has received grants or contracts from Ansun, Astellas, AstraZeneca, Eli Lilly, Enanta Pharmaceuticals, Genentech, Gilead, Janssen, Karius, Melinta, Merck, Moderna, Nabriva, Paratek, Pfizer, and Tetraphase; consulting fees from Finley Law Firm and Seqirus; payment or honoraria from Contemporary Pediatrics; and has participated in data safety monitoring boards or advisory boards for Adagio Therapeutics and ProventionBio. FC is a member of the Paediatric Committee at the European Medicines Agency (EMA), but has not participated in the deliberations or decisions related to this product (as communicated to the EMA). JBD has received consulting fees from Sanofi; payment or honoraria from Sanofi; and has participated in data safety monitoring boards or advisory boards for AstraZeneca. HJZ has received grants or contracts from AstraZeneca, MSD, and Pfizer; payment or honoraria from Sanofi; and has participated in data safety monitoring boards or advisory boards for Pfizer. AB, CC, MPG, TT, UWH, AL, and TV are employees of and hold stock or stock options in AstraZeneca. YY is a former employee of and holds stock or stock options in AstraZeneca. All other authors declare no competing interests.
Figures

Comment in
-
RSV immunisation: lessons from the COVID-19 pandemic.Lancet Child Adolesc Health. 2023 Mar;7(3):147-149. doi: 10.1016/S2352-4642(22)00377-7. Epub 2023 Jan 9. Lancet Child Adolesc Health. 2023. PMID: 36634693 No abstract available.
-
Will nirsevimab be the holy grail for prevention of respiratory syncytial virus lower respiratory tract infections in infants?Transl Pediatr. 2024 Mar 27;13(3):525-529. doi: 10.21037/tp-23-534. Epub 2024 Mar 8. Transl Pediatr. 2024. PMID: 38590379 Free PMC article. No abstract available.
References
-
- Hall CB. The burgeoning burden of respiratory syncytial virus among children. Infect Disord Drug Targets. 2012;12:92–97. - PubMed
-
- Rha B, Curns AT, Lively JY, et al. Respiratory syncytial virus-associated hospitalizations among young children: 2015–2016. Pediatrics. 2020;146 - PubMed
-
- Chaw PS, Hua L, Cunningham S, et al. Respiratory syncytial virus-associated acute lower respiratory infections in children with bronchopulmonary dysplasia: systematic review and meta-analysis. J Infect Dis. 2020;222(suppl 7):S620–S627. - PubMed
-
- Chaw PS, Wong SWL, Cunningham S, et al. Acute lower respiratory infections associated with respiratory syncytial virus in children with underlying congenital heart disease: systematic review and meta-analysis. J Infect Dis. 2020;222(suppl 7):S613–S619. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

