Microglia and meningeal macrophages depletion delays the onset of experimental autoimmune encephalomyelitis
- PMID: 36635255
- PMCID: PMC9835747
- DOI: 10.1038/s41419-023-05551-3
Microglia and meningeal macrophages depletion delays the onset of experimental autoimmune encephalomyelitis
Abstract
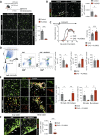
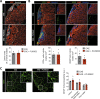
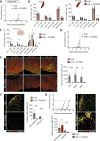
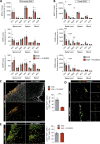
In multiple sclerosis and the experimental autoimmune encephalomyelitis (EAE) model, both resident microglia and infiltrating macrophages contribute to demyelination as well as spontaneous remyelination. Nevertheless, the specific roles of microglia versus macrophages are unknown. We investigated the influence of microglia in EAE using the colony stimulating factor 1 receptor (CSF-1R) inhibitor, PLX5622, to deplete microglial population and Ccr2RFP/+ fmsEGFP/+ mice, to distinguish blood-derived macrophages from microglia. PLX5622 treatment depleted microglia and meningeal macrophages, and provoked a massive infiltration of CCR2+ macrophages into demyelinating lesions and spinal cord parenchyma, albeit it did not alter EAE chronic phase. In contrast, microglia and meningeal macrophages depletion reduced the expression of major histocompatibility complex II and CD80 co-stimulatory molecule in dendritic cells, macrophages and microglia. In addition, it diminished T cell reactivation and proliferation in the spinal cord parenchyma, inducing a significant delay in EAE onset. Altogether, these data point to a specific role of CNS microglia and meningeal macrophages in antigen presentation and T cell reactivation at initial stages of EAE.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci 2017 202. 2017;20:136–44. - PubMed
-
- Rasmussen S, Wang Y, Kivisäkk P, Bronson RT, Meyer M, Imitola J, et al. Persistent activation of microglia is associated with neuronal dysfunction of callosal projecting pathways and multiple sclerosis-like lesions in relapsing-remitting experimental autoimmune encephalomyelitis. Brain. 2007;130:2816–29. doi: 10.1093/brain/awm219. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

