Hippo pathway dysregulation in gastric cancer: from Helicobacter pylori infection to tumor promotion and progression
- PMID: 36635265
- PMCID: PMC9837097
- DOI: 10.1038/s41419-023-05568-8
Hippo pathway dysregulation in gastric cancer: from Helicobacter pylori infection to tumor promotion and progression
Abstract
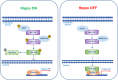
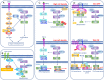
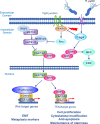
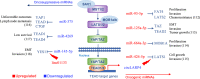
The Hippo pathway plays a critical role for balancing proliferation and differentiation, thus regulating tissue homeostasis. The pathway acts through a kinase cascade whose final effectors are the Yes-associated protein (YAP) and its paralog transcriptional co‑activator with PDZ‑binding motif (TAZ). In response to a variety of upstream signals, YAP and TAZ activate a transcriptional program that modulates cellular proliferation, tissue repair after injury, stem cell fate decision, and cytoskeletal reorganization. Hippo pathway signaling is often dysregulated in gastric cancer and in Helicobacter pylori-induced infection, suggesting a putative role of its deregulation since the early stages of the disease. In this review, we summarize the architecture and regulation of the Hippo pathway and discuss how its dysregulation fuels the onset and progression of gastric cancer. In this setting, we also focus on the crosstalk between Hippo and other established oncogenic signaling pathways. Lastly, we provide insights into the therapeutic approaches targeting aberrant YAP/TAZ activation and discuss the related clinical perspectives and challenges.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The Hippo Kinase LATS2 Controls Helicobacter pylori-Induced Epithelial-Mesenchymal Transition and Intestinal Metaplasia in Gastric Mucosa.Cell Mol Gastroenterol Hepatol. 2020;9(2):257-276. doi: 10.1016/j.jcmgh.2019.10.007. Epub 2019 Oct 24. Cell Mol Gastroenterol Hepatol. 2020. PMID: 31669263 Free PMC article.
-
Integrin-Linked Kinase in the Development of Gastric Tumors Induced by Helicobacter pylori: Regulation and Prevention Potential.Helicobacter. 2024 Jul-Aug;29(4):e13109. doi: 10.1111/hel.13109. Helicobacter. 2024. PMID: 38951739
-
Role of the Hippo pathway and mechanisms for controlling cellular localization of YAP/TAZ.FEBS J. 2022 Oct;289(19):5798-5818. doi: 10.1111/febs.16091. Epub 2021 Jul 13. FEBS J. 2022. PMID: 34173335 Review.
-
Role of Hippo pathway dysregulation from gastrointestinal premalignant lesions to cancer.J Transl Med. 2024 Feb 29;22(1):213. doi: 10.1186/s12967-024-05027-8. J Transl Med. 2024. PMID: 38424512 Free PMC article. Review.
-
TAZ Controls Helicobacter pylori-Induced Epithelial-Mesenchymal Transition and Cancer Stem Cell-Like Invasive and Tumorigenic Properties.Cells. 2020 Jun 13;9(6):1462. doi: 10.3390/cells9061462. Cells. 2020. PMID: 32545795 Free PMC article.
Cited by
-
Hippo Signaling Pathway in Colorectal Cancer: Modulation by Various Signals and Therapeutic Potential.Anal Cell Pathol (Amst). 2024 Oct 11;2024:5767535. doi: 10.1155/2024/5767535. eCollection 2024. Anal Cell Pathol (Amst). 2024. PMID: 39431199 Free PMC article. Review.
-
Regulation of Hippo/YAP axis in colon cancer progression by the deubiquitinase JOSD1.Cell Death Discov. 2024 Aug 14;10(1):365. doi: 10.1038/s41420-024-02136-7. Cell Death Discov. 2024. PMID: 39143074 Free PMC article.
-
CS-NO suppresses inhibits glycolysis and gastric cancer progression through regulating YAP/TAZ signaling pathway.Cell Biochem Biophys. 2023 Sep;81(3):561-567. doi: 10.1007/s12013-023-01153-0. Epub 2023 Aug 10. Cell Biochem Biophys. 2023. PMID: 37558859
-
Update on molecular pathogenesis of Helicobacter pylori-induced gastric cancer.World J Gastrointest Pathophysiol. 2025 Jun 22;16(2):107052. doi: 10.4291/wjgp.v16.i2.107052. World J Gastrointest Pathophysiol. 2025. PMID: 40568035 Free PMC article. Review.
-
YAP Activation Is Associated with a Worse Prognosis of Poorly Cohesive Gastric Cancer.J Pers Med. 2023 Aug 24;13(9):1294. doi: 10.3390/jpm13091294. J Pers Med. 2023. PMID: 37763062 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

