Effects of tuberculosis and/or HIV-1 infection on COVID-19 presentation and immune response in Africa
- PMID: 36635274
- PMCID: PMC9836341
- DOI: 10.1038/s41467-022-35689-1
Effects of tuberculosis and/or HIV-1 infection on COVID-19 presentation and immune response in Africa
Abstract
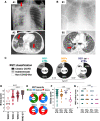
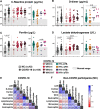
Few studies from Africa have described the clinical impact of co-infections on SARS-CoV-2 infection. Here, we investigate the presentation and outcome of SARS-CoV-2 infection in an African setting of high HIV-1 and tuberculosis prevalence by an observational case cohort of SARS-CoV-2 patients. A comparator group of non SARS-CoV-2 participants is included. The study includes 104 adults with SARS-CoV-2 infection of whom 29.8% are HIV-1 co-infected. Two or more co-morbidities are present in 57.7% of participants, including HIV-1 (30%) and active tuberculosis (14%). Amongst patients dually infected by tuberculosis and SARS-CoV-2, clinical features can be typical of either SARS-CoV-2 or tuberculosis: lymphopenia is exacerbated, and some markers of inflammation (D-dimer and ferritin) are further elevated (p < 0.05). Amongst HIV-1 co-infected participants those with low CD4 percentage strata exhibit reduced total, but not neutralising, anti-SARS-CoV-2 antibodies. SARS-CoV-2 specific CD8 T cell responses are present in 35.8% participants overall but undetectable in combined HIV-1 and tuberculosis. Death occurred in 30/104 (29%) of all COVID-19 patients and in 6/15 (40%) of patients with coincident SARS-CoV-2 and tuberculosis. This shows that in a high incidence setting, tuberculosis is a common co-morbidity in patients admitted to hospital with COVID-19. The immune response to SARS-CoV-2 is adversely affected by co-existent HIV-1 and tuberculosis.
© 2023. The Author(s).
Conflict of interest statement
All authors have completed the Unified Competing Interest form (available on request from the corresponding author) and declare: no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work.
Figures



References
-
- Sykes, W., et al. Prevalence of anti-SARS-CoV-2 antibodies among blood donors in Northern Cape, KwaZulu-Natal, Eastern Cape, and Free State provinces of South Africa in January 2021. Research Square, 10.21203/rs.21203.rs-233375/v233371 (2021).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

