Glomerulonephritis: immunopathogenesis and immunotherapy
- PMID: 36635359
- PMCID: PMC9838307
- DOI: 10.1038/s41577-022-00816-y
Glomerulonephritis: immunopathogenesis and immunotherapy
Abstract
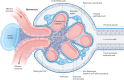
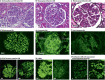
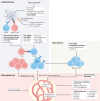
'Glomerulonephritis' (GN) is a term used to describe a group of heterogeneous immune-mediated disorders characterized by inflammation of the filtration units of the kidney (the glomeruli). These disorders are currently classified largely on the basis of histopathological lesion patterns, but these patterns do not align well with their diverse pathological mechanisms and hence do not inform optimal therapy. Instead, we propose grouping GN disorders into five categories according to their immunopathogenesis: infection-related GN, autoimmune GN, alloimmune GN, autoinflammatory GN and monoclonal gammopathy-related GN. This categorization can inform the appropriate treatment; for example, infection control for infection-related GN, suppression of adaptive immunity for autoimmune GN and alloimmune GN, inhibition of single cytokines or complement factors for autoinflammatory GN arising from inborn errors in innate immunity, and plasma cell clone-directed or B cell clone-directed therapy for monoclonal gammopathies. Here we present the immunopathogenesis of GN and immunotherapies in use and in development and discuss how an immunopathogenesis-based GN classification can focus research, and improve patient management and teaching.
© 2023. Springer Nature Limited.
Conflict of interest statement
H.-J.A. has received consultancy or lecture fees from Boehringer, Bayer, GlaxoSmithKline, AstraZeneca, Novartis, Otsuka, Janssen, Kezar, Lilly and PreviPharma. A.R.K. has received lecture fees from Vifor Pharma and research funding via consultancy and grants from Vifor, Visterra, Toleranzia, Variant Bio and CSL Limited. N.L. has received research funding from Omeros. P.R. has nothing to disclose.
Figures

References
-
- United States Renal Data System. 2022 annual data report. https://usrds-adr.niddk.nih.gov/2022?dkrd=/about-niddk/strategic-plans-r... (2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

