The prevalence of sepsis-induced coagulopathy in patients with sepsis - a secondary analysis of two German multicenter randomized controlled trials
- PMID: 36635426
- PMCID: PMC9837358
- DOI: 10.1186/s13613-022-01093-7
The prevalence of sepsis-induced coagulopathy in patients with sepsis - a secondary analysis of two German multicenter randomized controlled trials
Abstract
Background: Sepsis and septic shock are frequently accompanied by coagulopathy. Since the sepsis-induced coagulopathy (SIC) score was first described, subsequent studies from Asia revealed a SIC prevalence of 40-60%. In Europe, however, SIC prevalence in patients fulfilling sepsis criteria according to the third international consensus definition (SEPSIS-3) has not yet been evaluated.
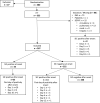
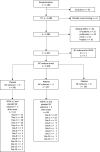
Methods: The Critical Care Trials Group of the German Sepsis Competence Network (SepNet) conducted a secondary analysis of two randomized controlled trials. Only patients fulfilling sepsis criteria according SEPSIS-3 were included in this secondary analysis. In a two step approach, SIC prevalence was determined in 267 patients with sepsis but not septic shock (at the time of inclusion) from the "Effect of Hydrocortisone on Development of Shock Among Patients With Severe Sepsis" (HYPRESS) trial. Then, we estimated SIC prevalence in 1,018 patients from the "Effect of Sodium Selenite Administration and Procalcitonin-Guided Therapy on Mortality in Patients With Severe Sepsis or Septic Shock" (SISPCT) trial using a simplified SIC score based on the platelet-SIC-subscore (PSSC). Study aims were to assess (i) the prevalence of SIC in patients with SEPSIS-3, (ii) the association of SIC with 90-day mortality and morbidity, (iii) the time when patients become SIC positive during the course of sepsis, and (iv) the value of the PSSC for predicting SIC.
Results: In the HYPRESS trial, SIC prevalence was 22.1% (95% confidence interval [CI] 17.5-27.5%). The estimated SIC prevalence in the SISPCT trial was 24.2% (95% CI 21.6-26.9%). In the HYPRESS trial, SIC was associated with significantly higher 90-day mortality (13.9% vs. 26.8%, p = 0.027) and morbidity. Logistic regression analysis adjusted for age, sex, treatment arm, and (SIC-adapted) SOFA score confirmed the negative association of SIC with survival (p = 0.011). In the SISPCT trial, increased PSSCs were associated with higher 90-day mortality (PSSC 0: 34.4%, PSSC 1: 40.5%, PSSC 2: 53.3%; p < 0.001). In both trials, SIC was already present at sepsis diagnosis or occurred during the following 4 days.
Conclusions: SIC is a clinically relevant complication of sepsis. Although it might be less frequent than previously reported, its occurrence is associated with higher morbidity and mortality and should be interpreted as an early warning sign.
Keywords: Platelet count; Prevalence; Sepsis; Sepsis-induced coagulopathy; Septic shock.
© 2023. The Author(s).
Conflict of interest statement
T. Schmoch reports that he has received lecture fees from Mitsubishi Tanabe Pharma GmbH, CSL Behring GmbH (Germany). T. Brenner reports that he has received honoraria for lectures or advisory boards: Baxter Deutschland GmbH, Schöchl medical education GmbH (Germany), Boehringer Ingelheim Pharma GmbH (Germany), CSL Behring GmbH (Germany), Astellas Pharma GmbH (Germany), B. Braun Melsungen AG (Germany), Lücke Kongresse GmbH (Germany), Sedana medical Germany GmbH (Germany), Shionogi GmbH (Germany) and MSD Sharp & Dohme GmbH (Germany) and advisory board and consulting activity for Baxter Deutschland GmbH (Germany). Furthermore, he received research funding from Deutsche Forschungsgemeinschaft (DFG), Dietmar Hopp Stiftung, Innovationsfonds of the Gemeinsamer Bundesausschuss (G-BA), and Stiftung Universitätsmedizin Essen. Patrick Möhnle reports that he has received reimbursement of conference fees and travel and accommodation expenses, and lecture fees from Bayer (Germany), Biotest (Germany), CSL Behring (Germany), NovoNordisk (Germany), Pfizer (Germany), Roche (Germany), Shire/Takeda, and SOBI (Germany) and advisory board member at CSL Behring (Germany). M.A. Weigand reports that he has received lecture fees from GE Healthcare (Germany), Gilead (Germany), Köhler Chemie (Germany), MSD Sharp & Dohme (Germany), Pfizer Pharma (Germany), and Boehringer Ingelheim (Germany) and was member of advisory boards at B. Braun (Germany), Gilead (Germany), MSD Sharp & Dohme (Germany), and Shionogi (Germany). P. Meybohm and/or the Department of Anesthesiology, Intensive Care, Emergency and Pain Medicine, University Hospital Wuerzburg (Wuerzburg, Germany) received research grants from the German Research Foundation (ME 3559/1-1, ME 3559/3-1), BMBF (01KG1815), BMG (ZMVI1-2520DAT10E); grants from B. Braun Melsungen, CSL Behring, Fresenius Kabi, and Vifor Pharma for the implementation of Frankfurt’s Patient Blood Management Program; honoraria for scientific lectures from Biotest AG, Vifor Pharma, CSL Behring, and Pharmacosmos. Michael Bauer, Frank Bloos, Holger Bogatsch, Josef Briegel, Gunnar Elke, Didier Keh and Markus Löffler declare that they have no competing interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

