Outcomes for the first four lines of therapy in patients with HER2-positive advanced breast cancer: results from the SONABRE registry
- PMID: 36635428
- PMCID: PMC10020272
- DOI: 10.1007/s10549-022-06832-9
Outcomes for the first four lines of therapy in patients with HER2-positive advanced breast cancer: results from the SONABRE registry
Abstract
Purpose: We assessed the systemic treatment choices and outcomes in patients diagnosed with human epidermal growth factor receptor-2-positive (HER2 +) advanced breast cancer (ABC), for the first four lines of systemic therapy and by hormone receptor (HR) status.
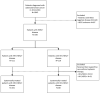
Methods: We identified 330 patients diagnosed with HER2 + ABC in 2013-2018 in the Southeast of The Netherlands, of whom 64% with HR + /HER2 + and 36% with HR-/HER2 + disease. Overall survival (OS) from start of therapy was calculated using the Kaplan-Meier method.
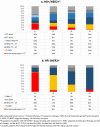
Results: In real world, 95% of patients with HR + /HER2 + and 74% of patients with HR-/HER2 + disease received systemic therapy. In HR + /HER2 + disease, use of endocrine, chemo- and HER2-targeted therapy was , respectively, 64%, 46% and 60% in first line, and 39%, 64% and 75% in fourth line. In HR-/HER2 + disease, 91-96% of patients received chemotherapy and 77-91% HER2-targeted therapy, irrespective of line of therapy. In patients with HR + /HER2 + disease, median OS was 34.9 months (95%CI:25.8-44.0) for the first line and 12.8 months (95%CI:10.7-14.9) for the fourth line. In HR-/HER2 + disease, median OS was 39.9 months (95%CI:23.9-55.8) for the first line and 15.2 months (95%CI:10.9-19.5) for the fourth line. For patients treated with first-line pertuzumab, trastuzumab plus chemotherapy, median OS was not reached at 56.0 months in HR + /HER2 + disease and 48.4 months (95%CI:32.6-64.3) in HR-/HER2 + disease.
Conclusion: Survival times for later lines of therapy are surprisingly long and justify the use of multiple lines of systemic therapy in well-selected patients with HER2 + ABC. Our real-world evidence adds valuable observations to the accumulating evidence that within HER2 + ABC, the HR status defines two distinct disease subtypes.
Keywords: Breast neoplasms; ERBB2 protein; Neoplasm metastasis; Pertuzumab; Registries; Treatment Outcome.
© 2023. The Author(s).
Conflict of interest statement
Khava I.E. Ibragimova Financial interests: Grants from Novartis BV, Roche, Pfizer, Eli Lilly, Daiichi-Sankyo. Marissa Meegdes Financial interests: Grants from Novartis BV, Roche, Pfizer, Eli Lilly, Daiichi-Sankyo. Sandra M.E. Geurts Financial interests: Grants from Novartis BV, Roche, Pfizer, Eli Lilly, Daiichi-Sankyo, Gilead, Grants and personal fees from AstraZeneca. Nathalie J.A. Teeuwen-Dedroog Financial interests: Grants from Novartis BV, Roche, Pfizer, Eli Lilly, Daiichi-Sankyo. Ingeborg Vriens Financial interests: Grants from AstraZeneca and Pfizer. Vivianne C.G. Tjan-Heijnen Financial interests: Grants and personal fees from Roche, grants and personal fees from Novartis, grants and personal fees from Pfizer, grants and personal fees from Lilly, grants and personal fees from AstraZeneca, grants from Daiichi-Sankyo and grants from Gilead. All remaining authors have no relevant financial or non-financial interests to disclose. Signed by first author Khava Ibragimova on behalf of all authors.
Figures


References
-
- Blackwell KL, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol. 2012;30(21):2585–2592. doi: 10.1200/JCO.2011.35.6725. - DOI - PubMed
-
- (EMA), E.M.A., Perjeta (pertuzumab) autorisation details. https://www.ema.europa.eu/, 2013.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

