Measuring Nonapoptotic Caspase Activity with a Transgenic Reporter in Mice
- PMID: 36635920
- PMCID: PMC9536855
- DOI: 10.1523/ENEURO.0147-21.2022
Measuring Nonapoptotic Caspase Activity with a Transgenic Reporter in Mice
Abstract
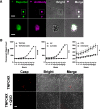
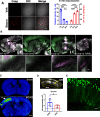
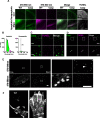
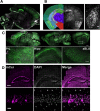
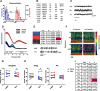
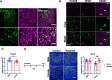
The protease caspase-3 is a key mediator of apoptotic programmed cell death. But weak or transient caspase activity can contribute to neuronal differentiation, axonal pathfinding, and synaptic long-term depression. Despite the importance of sublethal, or nonapoptotic, caspase activity in neurodevelopment and neural plasticity, there has been no simple method for mapping and quantifying nonapoptotic caspase activity (NACA) in rodent brains. We therefore generated a transgenic mouse expressing a highly sensitive and specific fluorescent reporter of caspase activity, with peak signal localized to the nucleus. As a proof of concept, we first obtained evidence that NACA influences neurophysiology in an amygdalar circuit. Then focusing on the amygdala, we were able to quantify a sex-specific persistent elevation in caspase activity in females after restraint stress. This simple in vivo caspase activity reporter will facilitate systems-level studies of apoptotic and nonapoptotic phenomena in behavioral and pathologic models.
Keywords: amygdala; caspases; in vivo reporter; mapping; nonapoptotic; sex differences; stress.
Copyright © 2022 Nicholls et al.
Figures

References
-
- Beevor C (1883) Die Kleinhirnrinde. Arch Amt Physiol 365–388.
-
- Beraldo FH, Arantes CP, Santos TG, Queiroz NG, Young K, Rylett RJ, Markus RP, Prado MA, Martins VR (2010) Role of alpha7 nicotinic acetylcholine receptor in calcium signaling induced by prion protein interaction with stress-inducible protein 1. J Biol Chem 285:36542–36550. 10.1074/jbc.M110.157263 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
