Binding and neutralizing anti-AAV antibodies: Detection and implications for rAAV-mediated gene therapy
- PMID: 36635967
- PMCID: PMC10014285
- DOI: 10.1016/j.ymthe.2023.01.010
Binding and neutralizing anti-AAV antibodies: Detection and implications for rAAV-mediated gene therapy
Abstract
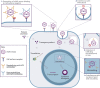
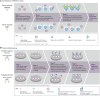
Assessment of anti-adeno-associated virus (AAV) antibodies in patients prior to systemic gene therapy administration is an important consideration regarding efficacy and safety of the therapy. Approximately 30%-60% of individuals have pre-existing anti-AAV antibodies. Seroprevalence is impacted by multiple factors, including geography, age, capsid serotype, and assay type. Anti-AAV antibody assays typically measure (1) transduction inhibition by detecting the neutralizing capacity of antibodies and non-antibody neutralizing factors, or (2) total anti-capsid binding antibodies, regardless of neutralizing activity. Presently, there is a paucity of head-to-head data and standardized approaches associating assay results with clinical outcomes. In addition, establishing clinically relevant screening titer cutoffs is complex. Thus, meaningful comparisons across assays are nearly impossible. Although complex, establishing screening assays in routine clinical practice to identify patients with antibody levels that may impact favorable treatment outcomes is achievable for both transduction inhibition and total antibody assays. Formal regulatory approval of such assays as companion diagnostic tests will confirm their suitability for specific recombinant AAV gene therapies. This review covers current approaches to measure anti-AAV antibodies in patient plasma or serum, their potential impact on therapeutic safety and efficacy, and investigative strategies to mitigate the effects of pre-existing anti-AAV antibodies in patients.
Keywords: efficacy; gene therapy; humoral immunity; neutralizing antibodies; recombinant adeno-associated virus; safety; seroprevalence; total antibody assay; transduction inhibition assay.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests S.H.C., M.S., S.S., G.B., I.W., M.M., and D.L. are employees of Pfizer. C.J.P. is an employee of Labcorp-Monogram Biosciences.
Figures



References
-
- US Food and Drug Administration What is gene therapy? 2018. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-produ...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

