Congenital cardiac liver cirrhosis with combined hepatocellular-cholangiocarcinoma-a case report
- PMID: 36636063
- PMCID: PMC9830364
- DOI: 10.21037/jgo-21-878
Congenital cardiac liver cirrhosis with combined hepatocellular-cholangiocarcinoma-a case report
Abstract
Background: Cardiac liver cirrhosis secondary to Fontan procedure has been associated with hepatocellular carcinoma at a younger age. However, Fontan associated liver disease and combined hepatocellular-cholangiocarcinoma has not been previously reported. Combined hepatocellular-cholangiocarcinoma is a rare cancer that accounts for 2-5% of primary liver tumors and poses significant diagnostic and treatment challenges. This case highlights these needs and potential screening and treatment considerations. Herein we describe a case of combined hepatocellular-cholangiocarcinoma in a patient with autism, congenital heart disease, and Fontan procedure.
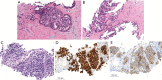
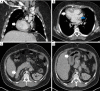
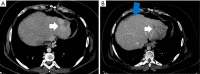
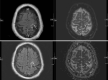
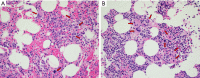
Case description: The patient is a 27-year-old male who presented with a liver mass detected on MRI performed in the context of a rising alpha-fetoprotein during a screening visit. Biopsy of the mass revealed a combined hepatocellular-cholangiocarcinoma which was staged as localized. Due to the COVID-19 pandemic and subsequent halt of all elective surgeries, the patient received local therapy with chemoembolization followed by pembrolizumab. The disease progressed though, and therapy was changed to gemcitabine plus cisplatin. Patient received 2 cycles of therapy, after which he and his family decided to transfer medical care to Memorial Sloan Kettering. Next generation sequencing of the tumor revealed TP53 and FGFR2 mutations. By then patient was also found to have lung metastasis. To help address the hepatocellular carcinoma, lenvatinib was added. Patient had sustainable disease control for about a year, yet eventually developed thrombocytopenia complicated by an episode of gastrointestinal bleeding. With a worsening performance status, adverse events of the treatment, and recurrent hospitalizations, a goals of care discussion with his family led to the discontinuation of active cancer therapy and patient was started on best supportive care. Patient remained in active follow-up until the time of this report and passed away less than a year from initiating best supportive care alone.
Conclusions: This challenging case raises awareness towards screening and monitoring all patients with Fontan procedure for Fontan associated liver disease and liver cancers, including combined hepatocellular-cholangiocarcinoma. To the best of our knowledge, this is the first description of combined hepatocellular-cholangiocarcinoma occurring in the context of cardiac cirrhosis. The management difficulties that led to altering the goals of care, is another reminder of the dynamic nature of the care oncologists would provide.
Keywords: Fontan; cardiac cirrhosis; case report; combined hepatocellular-cholangiocarcinoma.
2022 Journal of Gastrointestinal Oncology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available a https://jgo.amegroups.com/article/view/10.21037/jgo-21-878/coif). DM reports that she received honoraria from Astra Zeneca for lectures, presentations, and educational events. WB reports that he received royalties from Oxford University Press Royalty textbooks and Kluwer Publishing, consulting fees from Blue Note Therapeutics, and honoraria from Kubler Ross Foundation of Argentina for lecture, GKA reports that he received support from Adicet, Alnylam, AstraZeneca, Autem, Beigene, Berry Genomics, Boehringer Ingelheim, Celgene, Cend, CytomX, Eisai, Eli Lilly, Exelixis,Flatiron, Genentech/Roche, Genoscience, Helio, Helsinn, Incyte, Ipsen, Merck, Nerviano, Newbridge, Novartis, QED, Redhill, Rafael, Servier, Silenseed, Sobi, Vector, Yiviva for consultancy and from Arcus, Astra Zeneca, BioNtech, BMS, Celgene, Flatiron, Genentech/Roche, Genoscience, Incyte, Polaris, Puma, QED, Silenseed, Yiviva for institutional grants. The other authors have no conflicts of interest to declare.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
