The roles of Runx1 in skeletal development and osteoarthritis: A concise review
- PMID: 36636224
- PMCID: PMC9830174
- DOI: 10.1016/j.heliyon.2022.e12656
The roles of Runx1 in skeletal development and osteoarthritis: A concise review
Abstract
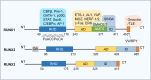
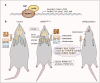
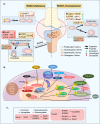
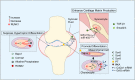
Runt-related transcription factor-1 (Runx1) is well known for its functions in hematopoiesis and leukemia but recent research has focused on its role in skeletal development and osteoarthritis (OA). Deficiency of the Runx1 gene is fatal in early embryonic development, and specific knockout of Runx1 in cell lineages of cartilage and bone leads to delayed cartilage formation and impaired bone calcification. Runx1 can regulate genes including collagen type II (Col2a1) and X (Col10a1), SRY-box transcription factor 9 (Sox9), aggrecan (Acan) and matrix metalloproteinase 13 (MMP-13), and the up-regulation of Runx1 improves the homeostasis of the whole joint, even in the pathological state. Moreover, Runx1 is activated as a response to mechanical compression, but impaired in the joint with the pathological progress associated with osteoarthritis. Therefore, interpretation about the role of Runx1 could enlarge our understanding of key marker genes in the skeletal development and an increased understanding of Runx1 could be helpful to identify treatments for osteoarthritis. This review provides the most up-to-date advances in the roles and bio-mechanisms of Runx1 in healthy joints and osteoarthritis from all currently published articles and gives novel insights in therapeutic approaches to OA based on Runx1.
Keywords: Bone; Chondrogenesis; Gene therapy; Osteoarthritis; Osteogenesis; Runx1; Skeletal development.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Runx1 protects against the pathological progression of osteoarthritis.Bone Res. 2021 Dec 7;9(1):50. doi: 10.1038/s41413-021-00173-x. Bone Res. 2021. PMID: 34876557 Free PMC article.
-
Runx1 contributes to articular cartilage maintenance by enhancement of cartilage matrix production and suppression of hypertrophic differentiation.Sci Rep. 2019 May 21;9(1):7666. doi: 10.1038/s41598-019-43948-3. Sci Rep. 2019. PMID: 31113964 Free PMC article.
-
Possible roles of Runx1 and Sox9 in incipient intramembranous ossification.J Bone Miner Res. 2004 Oct;19(10):1671-7. doi: 10.1359/JBMR.040801. Epub 2004 Aug 3. J Bone Miner Res. 2004. PMID: 15355562
-
[Latest Findings on the Role of RUNX1 in Bone Development and Disorders].Sichuan Da Xue Xue Bao Yi Xue Ban. 2024 Mar 20;55(2):256-262. doi: 10.12182/20240360103. Sichuan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38645858 Free PMC article. Review. Chinese.
-
[RESEARCH PROGRESS OF PATHOLOGY OF ENDOCHONDRAL OSSIFICATION IN OSTEOARTHRITIS].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016 Dec 8;30(12):1556-1561. doi: 10.7507/1002-1892.20160320. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016. PMID: 29786351 Review. Chinese.
Cited by
-
Osteoarthritis: Mechanisms and Therapeutic Advances.MedComm (2020). 2025 Aug 1;6(8):e70290. doi: 10.1002/mco2.70290. eCollection 2025 Aug. MedComm (2020). 2025. PMID: 40757100 Free PMC article. Review.
-
RUN(X) out of blood: emerging RUNX1 functions beyond hematopoiesis and links to Down syndrome.Hum Genomics. 2023 Sep 5;17(1):83. doi: 10.1186/s40246-023-00531-2. Hum Genomics. 2023. PMID: 37670378 Free PMC article. Review.
-
Regulation of Biomineralization and Autophagy by the Stress-Sensing Transcription Factor CgRunx1 in Crassostrea gigas Under Daylight Ultraviolet B Radiation.Mar Biotechnol (NY). 2024 Dec;26(6):1260-1270. doi: 10.1007/s10126-024-10370-4. Epub 2024 Sep 5. Mar Biotechnol (NY). 2024. PMID: 39235651
-
Bioinformatics and systems biology approaches to identify potential common pathogeneses for sarcopenia and osteoarthritis.Front Med (Lausanne). 2024 Jun 18;11:1380210. doi: 10.3389/fmed.2024.1380210. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38962732 Free PMC article.
References
-
- Yoshida C.A., Komori T. Role of Runx proteins in chondrogenesis. Crit. Rev. Eukaryot. Gene Expr. 2005;15:243–254. - PubMed
-
- Wang Y., Belflower R.M., Dong Y.F., Schwarz E.M., O'Keefe R.J., Drissi H. Runx1/AML1/Cbfa2 mediates onset of mesenchymal cell differentiation toward chondrogenesis. J. Bone Miner. Res. 2005;20:1624–1636. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

