Adjuvant chemotherapy for completely resected IIA-IIIA non-small cell lung cancer: compliance to guidelines, safety and efficacy in real-life practice
- PMID: 36636405
- PMCID: PMC9830267
- DOI: 10.21037/tlcr-22-345
Adjuvant chemotherapy for completely resected IIA-IIIA non-small cell lung cancer: compliance to guidelines, safety and efficacy in real-life practice
Abstract
Background: Since randomised clinical trials demonstrated a survival benefit of adjuvant chemotherapy (AC) following curative-intent lung surgery, AC has been implemented as a standard therapeutic strategy for patients with a completely resected IIA-IIIA non-small cell lung cancer (NSCLC). Regarding the moderate benefit of AC and the lack of literature on AC use in real-life practice, we aimed to evaluate compliance to guidelines, AC safety and efficacy in a less selected population.
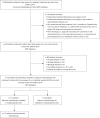
Methods: Between January 2009 and December 2014, we retrospectively analysed 210 patients with theoretical indication of AC following curative-intent lung surgery for a completely resected IIA-IIIA NSCLC. The primary objective of this retrospective study was to evaluate compliance to AC guidelines. Secondary objectives included safety and efficacy of AC in real-life practice.
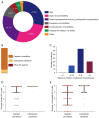
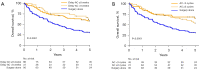
Results: Among 210 patients with a theoretical indication of AC, chemotherapy administration was validated in multidisciplinary team (MDT) for 62.4% of them and 117 patients (55.7%) finally received AC. Patient's clinical conditions were the main reasons advanced in MDT for no respect to AC guidelines. Most of the patients received cisplatin-vinorelbine (86.3%) and AC was initiated within 8 weeks following lung surgery for 73.5% of patients. One-half of patients who received AC experienced side effects leading to either dose-intensity modification or treatment interruption. In real-life practice, AC was found to provide a survival benefit over surgery alone. Factors related to daily-life practice such as delayed AC initiation or incomplete AC planned dose received were not associated with an inferior survival.
Conclusions: Although AC use might differ from guidelines in real-life practice, this retrospective study highlights that AC can be used safely and remains efficient among a less selected population. In the context of immunotherapy and targeted therapies development in peri-operative treatment strategies, the place of AC has to be precised in the future.
Keywords: Adjuvant chemotherapy (AC); non-small cell lung cancer (NSCLC); real-life practice.
2022 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-345/coif). The authors have no conflicts of interest to declare.
Figures

Comment in
-
Adjuvant chemotherapy for locally advanced non-small cell lung cancer: still state of the art or an outdated therapy?Transl Lung Cancer Res. 2023 Feb 28;12(2):204-206. doi: 10.21037/tlcr-22-888. Epub 2023 Jan 15. Transl Lung Cancer Res. 2023. PMID: 36895929 Free PMC article. No abstract available.
References
LinkOut - more resources
Full Text Sources
