Role of ctDNA for the detection of minimal residual disease in resected non-small cell lung cancer: a systematic review
- PMID: 36636413
- PMCID: PMC9830273
- DOI: 10.21037/tlcr-22-390
Role of ctDNA for the detection of minimal residual disease in resected non-small cell lung cancer: a systematic review
Abstract
Background: Operable stage I-III non-small cell lung cancer (NSCLC) has a high risk of recurrence, mainly due to remnant clones of the disease defined as minimal residual disease (MRD). Adjuvant chemotherapy has a limited efficacy in reducing the risk of relapse, and prognostic as well as predictive biomarkers in this context are currently missing.
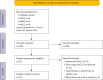
Methods: We performed a systematic review to evaluate the state of the art about the role of circulating tumor DNA detection through liquid biopsy for the assessment of MRD in resected early-stage NSCLC patients.
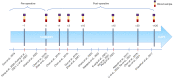
Results: Among the 650 studies identified, 13 were eligible and included. Although highly heterogeneous, all the studies demonstrated a poor prognosis in patients with post-operative MRD, with a detection rate ranging from 6% to 45%. MRD detection preceded radiographic/clinical recurrence by a mean of 5.5 months. MRD positive patients were most likely to benefit from adjuvant treatment in terms of recurrence-free survival (RFS). Consistently, adjuvant therapy did not minimize the risk of relapse in the MRD negative group.
Conclusions: Liquid biopsy has a relevant role in assessing post-surgical MRD in resected NSCLC. Since currently there are no criteria other than stage and risk factors for the choice of adjuvant treatment in this setting, post-operative assessment of MRD through liquid biopsy might be a promising approach to guide the decision.
Keywords: Non-small cell lung cancer (NSCLC); adjuvant therapy; circulating tumor DNA (ctDNA); minimal residual disease (MRD).
2022 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-390/coif). MT serves as an unpaid editorial board member of Translational Lung Cancer Research from December 2021 to November 2023. MT has received speakers’ and/or consultants’ fee from Astra-Zeneca, Pfizer, Eli-Lilly, BMS, Novartis, Roche, MSD, Boehringer Ingelheim, Takeda, Amgen, Merck, and Sanofi. MT received institutional research grants from Astra-Zeneca and Boehringer Ingelheim. AL has received speakers’ fees for Astra-Zeneca and MSD. AL has been on advisory boards for BeiGene and Sanofi. RM has received a payment for manuscript writing from Novartis. The other authors have no conflicts of interest to declare.
Figures

Comment in
-
Molecular minimal residual disease in resected non-small cell lung cancer (NSCLC): results of specifically designed interventional clinical trials eagerly awaited.Transl Lung Cancer Res. 2023 Feb 28;12(2):200-203. doi: 10.21037/tlcr-22-899. Epub 2023 Jan 19. Transl Lung Cancer Res. 2023. PMID: 36895928 Free PMC article. No abstract available.
Similar articles
-
Perioperative ctDNA-Based Molecular Residual Disease Detection for Non-Small Cell Lung Cancer: A Prospective Multicenter Cohort Study (LUNGCA-1).Clin Cancer Res. 2022 Aug 2;28(15):3308-3317. doi: 10.1158/1078-0432.CCR-21-3044. Clin Cancer Res. 2022. PMID: 34844976
-
Investigate the application of postoperative ctDNA-based molecular residual disease detection in monitoring tumor recurrence in patients with non-small cell lung cancer--A retrospective study of ctDNA.Front Oncol. 2023 Apr 6;13:1098128. doi: 10.3389/fonc.2023.1098128. eCollection 2023. Front Oncol. 2023. PMID: 37091156 Free PMC article.
-
Clinical application of minimal residual disease detection by ctDNA testing in non-small cell lung cancer: a narrative review.Transl Lung Cancer Res. 2025 Mar 31;14(3):1007-1020. doi: 10.21037/tlcr-24-942. Epub 2025 Mar 27. Transl Lung Cancer Res. 2025. PMID: 40248720 Free PMC article. Review.
-
Potential clinical utility of liquid biopsy in early-stage non-small cell lung cancer.BMC Med. 2022 Dec 14;20(1):480. doi: 10.1186/s12916-022-02681-x. BMC Med. 2022. PMID: 36514063 Free PMC article.
-
Detection of circulating tumor DNA with ultradeep sequencing of plasma cell-free DNA for monitoring minimal residual disease and early detection of recurrence in early-stage lung cancer.Cancer. 2024 May 15;130(10):1758-1765. doi: 10.1002/cncr.35263. Epub 2024 Feb 29. Cancer. 2024. PMID: 38422026
Cited by
-
A comprehensive overview of minimal residual disease in the management of early-stage and locally advanced non-small cell lung cancer.NPJ Precis Oncol. 2025 Jun 13;9(1):178. doi: 10.1038/s41698-025-00984-9. NPJ Precis Oncol. 2025. PMID: 40514443 Free PMC article.
-
The IASLC uncertain resection, general overview, current evidence, and future prospects: a systematic review and meta-analysis.Ther Adv Med Oncol. 2025 Jul 15;17:17588359251344789. doi: 10.1177/17588359251344789. eCollection 2025. Ther Adv Med Oncol. 2025. PMID: 40672739 Free PMC article. Review.
-
Values of circulating tumor DNA for non-small cell lung cancer patients receiving neoadjuvant therapy, progress and challenges: a narrative review.J Thorac Dis. 2024 Jul 30;16(7):4742-4755. doi: 10.21037/jtd-24-265. Epub 2024 Jul 4. J Thorac Dis. 2024. PMID: 39144303 Free PMC article. Review.
-
Perioperative immunotherapy in stage IB-III non-small cell lung cancer: a critical review of its rationale and considerations.Korean J Intern Med. 2023 Nov;38(6):787-796. doi: 10.3904/kjim.2023.345. Epub 2023 Nov 1. Korean J Intern Med. 2023. PMID: 37939663 Free PMC article. Review.
-
Challenges in the implementation of ultrasensitive liquid biopsy approaches in precision oncology.J Immunother Cancer. 2023 Jun;11(6):e006793. doi: 10.1136/jitc-2023-006793. J Immunother Cancer. 2023. PMID: 37349128 Free PMC article. No abstract available.
References
-
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Non-Small Cell Lung Cancer Version 2.2022 - March 7, 2022. Available online: http://www.nccn.org/guidelines/ (accessed on 11 March 2022).
Publication types
LinkOut - more resources
Full Text Sources
