Efficacy and safety of alectinib in ALK-positive non-small cell lung cancer and blood markers for prognosis and efficacy: a retrospective cohort study
- PMID: 36636415
- PMCID: PMC9830262
- DOI: 10.21037/tlcr-22-857
Efficacy and safety of alectinib in ALK-positive non-small cell lung cancer and blood markers for prognosis and efficacy: a retrospective cohort study
Abstract
Background: Alectinib is a second generation of ALK-tyrosine kinase inhibitors (ALK-TKIs), which has attracted much attention in the treatment of ALK-positive non-small cell lung cancer (NSCLC). At present, there are few reports on the efficacy and safety of alectinib in Chinese population. Moreover, biomarkers reflecting prognosis and efficacy are exceedingly needed. This study assessed the efficacy of alectinib in patients with ALK-positive NSCLC and analyzed the prognostic factors.
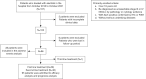
Methods: Patients with ALK-positive NSCLC who were confirmed by histopathology or cytology at the Affiliated Cancer Hospital of Nanjing Medical University between October 2018 and October 2021 were enrolled. All patients were treated with alectinib. The clinical characteristics and circulating tumor biomarkers before and after treatment were collected. Kaplan-Meier test was used to calculate the progression-free survival (PFS). Univariate and multivariate Cox regression analyses were used to explore the influencing factors on PFS. Incidence of adverse events was observed.
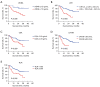
Results: Twenty patients progressed after first-line treatment (n=59) with alectinib, and 21 patients progressed following second-line treatment (n=36) with alectinib. The median PFS of first-line treatment patients was not achieved, and the median PFS of patients undergoing second-line treatment was 15.0 months [95% confidence interval (CI): 0.00-32.23]. The most common adverse reactions were liver dysfunction (37.50%), anemia (37.50%), and constipation (20.83%). The incidence of grade III and above adverse reactions was 6.25%. Univariate analysis showed that neutrophil-to-lymphocyte ratio [NLR; hazard ratio (HR) =0.424, P=0.005] carcinoembryonic antigen (CEA; HR =0.482, P=0.029), lactate dehydrogenase (LDH; HR =0.327, P<0.001), carbohydrate antigen (CA)199 (HR =0.313, P=0.002), and circulating cell free DNA (cfDNA; HR =0.229, P=0.008) concentration levels were associated with PFS, and multivariate analysis showed that NLR (HR =3.058, P=0.034) was independent prognostic factor. After three months of treatment, CEA, CA199, NLR, and LDH, could further predict the prognosis of alectinib treatment.
Conclusions: The efficacy and safety of alectinib as a first-line or second-line treatment for ALK-positive NSCLC in keeping with published prospective studies. CEA, CA199, NLR, and LDH within the normal range after three months of treatment were associated with good prognosis. Detection of serum tumor markers can indicate therapeutic success in patients treated with alectinib.
Keywords: ALK-positive; Non-small cell lung cancer (NSCLC); adverse drug reaction (ADR); alectinib; biomarkers.
2022 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-857/coif). AR declares consulting fees form AstraZeneca, participation on advisory board (Takeda), supporting for attending to meetings form Thermofisher and BMS. BGMH declares Advisory Board of Merck, Sharpe and Dohme, Eisai, Pfizer, Sanofi and Takeda. The other authors have no conflicts of interest to declare.
Figures






Similar articles
-
Effect of alectinib versus crizotinib on progression-free survival, central nervous system efficacy and adverse events in ALK-positive non-small cell lung cancer: a systematic review and meta-analysis.Ann Palliat Med. 2020 Jul;9(4):1782-1796. doi: 10.21037/apm-19-643. Epub 2020 Jun 8. Ann Palliat Med. 2020. PMID: 32527124
-
Analysis of the resistance profile of real-world alectinib first-line therapy in patients with ALK rearrangement-positive advanced non-small cell lung cancer using organoid technology in one case of lung cancer.J Thorac Dis. 2024 Jun 30;16(6):3854-3863. doi: 10.21037/jtd-23-1964. Epub 2024 Jun 14. J Thorac Dis. 2024. PMID: 38983150 Free PMC article.
-
Alectinib versus crizotinib in ALK-positive advanced non-small cell lung cancer and comparison of next-generation TKIs after crizotinib failure: Real-world evidence.Cancer Med. 2022 Dec;11(23):4491-4500. doi: 10.1002/cam4.4834. Epub 2022 May 26. Cancer Med. 2022. PMID: 35616090 Free PMC article.
-
Final progression-free survival results from the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer.Lung Cancer. 2020 Jan;139:195-199. doi: 10.1016/j.lungcan.2019.11.025. Epub 2019 Nov 28. Lung Cancer. 2020. PMID: 31812890
-
Mixed responses to first-line alectinib in non-small cell lung cancer patients with rare ALK gene fusions: A case series and literature review.J Cell Mol Med. 2021 Oct;25(19):9476-9481. doi: 10.1111/jcmm.16897. Epub 2021 Sep 19. J Cell Mol Med. 2021. PMID: 34541785 Free PMC article. Review.
Cited by
-
Tailoring Therapeutic Strategies in Non-Small-Cell Lung Cancer: The Role of Genetic Mutations and Programmed Death Ligand-1 Expression in Survival Outcomes.Cancers (Basel). 2023 Oct 31;15(21):5248. doi: 10.3390/cancers15215248. Cancers (Basel). 2023. PMID: 37958421 Free PMC article.
-
Evaluation of Solubility, Dissolution Rate, and Oral Bioavailability of β-Cyclodextrin and Hydroxypropyl β-Cyclodextrin as Inclusion Complexes of the Tyrosine Kinase Inhibitor, Alectinib.Pharmaceuticals (Basel). 2024 Jun 5;17(6):737. doi: 10.3390/ph17060737. Pharmaceuticals (Basel). 2024. PMID: 38931404 Free PMC article.
-
Organizing pneumonia in ALK+ non-small cell lung cancer treated with ceritinib: a case report.Discov Oncol. 2025 Jul 18;16(1):1363. doi: 10.1007/s12672-025-02904-6. Discov Oncol. 2025. PMID: 40679663 Free PMC article.
-
Clinicopathological characteristics correlated with programmed cell death-ligand 1 expression in advanced lung adenocarcinoma.J Thorac Dis. 2023 Oct 31;15(10):5307-5318. doi: 10.21037/jtd-23-523. Epub 2023 Sep 11. J Thorac Dis. 2023. PMID: 37969280 Free PMC article.
References
-
- Li H. Correlation between ALK,ROS1 and BRAF-V600E gene mutations and clinical features in patients with non-small cell lung carcinoma. Journal of Molecular Diagnostics and Therapy 2020;12:701-4,719.
LinkOut - more resources
Full Text Sources
Research Materials
