Atherosclerosis With Immune Checkpoint Inhibitor Therapy: Evidence, Diagnosis, and Management: JACC: CardioOncology State-of-the-Art Review
- PMID: 36636438
- PMCID: PMC9830225
- DOI: 10.1016/j.jaccao.2022.11.011
Atherosclerosis With Immune Checkpoint Inhibitor Therapy: Evidence, Diagnosis, and Management: JACC: CardioOncology State-of-the-Art Review
Abstract
As the clinical applications of immune checkpoint inhibitors (ICIs) expand, our knowledge of the potential adverse effects of these drugs continues to broaden. Emerging evidence supports the association between ICI therapy with accelerated atherosclerosis and atherosclerotic cardiovascular (CV) events. We discuss the biological plausibility and the clinical evidence supporting an effect of inhibition of these immune checkpoints on atherosclerotic CV disease. Further, we provide a perspective on potential diagnostic and pharmacological strategies to reduce atherosclerotic risk in ICI-treated patients. Our understanding of the pathophysiology of ICI-related atherosclerosis is in its early stages. Further research is needed to identify the mechanisms linking ICI therapy to atherosclerosis, leverage the insight that ICI therapy provides into CV biology, and develop robust approaches to manage the expanding cohort of patients who may be at risk for atherosclerotic CV disease.
Keywords: ASCVD, atherosclerotic cardiovascular disease; CTLA-4, cytotoxic T-lymphocyte associated protein 4; ICI, immune checkpoint inhibitor; IFN, interferon; IL, interleukin; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; TNF, tumor necrosis; atherosclerosis; cardio-oncology; cardiovascular disease; immune checkpoint inhibitor; inflammation; irAE, immune-related adverse event.
© 2022 The Authors.
Conflict of interest statement
Dr Neilan holds the Michael and Kathryn Park Endowed Chair in Cardiology and was also supported, in part, through a kind gift from A. Curtis Greer and Pamela Kohlberg and Christina and Paul Kazilionis, a Hassenfeld Scholar Award, and grants from the National Institutes of Health/National Heart, Lung, and Blood Institute (R01HL130539, R01HL159187, R01HL137562, K24HL150238). Dr Zanni was supported, in part, through grants from the National Institutes of Health/National Heart, Lung, and Blood Institute (R01HL146267 and R01HL137562) and from the National Institutes of Health/National Institute of Allergy and Infectious Diseases (K24AI157882); and has served as the principal investigator on an investigator-initiated research grant from Gilead to her institution (Massachusetts General Hospital). Dr Neilan has served as a consultant for and received fees from Amgen, Genentech, Roche, BMS, Sanofi, CRC Oncology, and AbbVie outside of the current work; and received grant funding from AstraZeneca and Bristol Myers Squibb related to immune checkpoint inhibitors. Dr Suero-Abreu has reported that she has no relationships relevant to the contents of this paper to disclose.
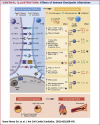
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

