Immune Checkpoint Inhibitor Myocarditis Treatment Strategies and Future Directions
- PMID: 36636442
- PMCID: PMC9830216
- DOI: 10.1016/j.jaccao.2022.11.005
Immune Checkpoint Inhibitor Myocarditis Treatment Strategies and Future Directions
Keywords: CD86RO, cluster of differentiation 86 receptor occupancy; CTLA-4, cytotoxic T-lymphocyte antigen; ICI, immune checkpoint inhibitor; JAK, Janus kinase; MACE, major adverse cardiac event; PD-1, programmed cell death 1 receptor; abatacept; immunotherapy; lung cancer; myocarditis; pharmacology.
Conflict of interest statement
Dr Moslehi is supported by National Institutes of Health grants (R01HL141466, R01HL155990, R01HL156021, and R01HL160688); and has served on advisory boards for Pfizer, Novartis, Bristol-Myers Squibb, Deciphera, Audentes Pharmaceuticals, Takeda, AstraZeneca, Heat Biologics, Incyte, Vertex, Verastem Oncology, Acceleron Pharmaceuticals, GlaxoSmithKline, TripleGene LLC, Regeneron, Nektar Therapeutics, Boston Biomedical, ImmunoCore, Janssen, Myovant, Silverback Therapeutics, Amgen, Kurome Therapeutics, Kiniksa Pharmaceuticals, Daiichi Sankyo, CRC Oncology, BeiGene, Star Therapeutics, ProteinQure, Pharmacyclics, Kiniksa, Prelude Therapeutics Mallinckrodt Pharmaceuticals, Boehringer, TransThera Sciences, Antev Ltd, IQVIA, AskBio, Lapcorp, Paladin, Quell Therapeutics, Voyager Therapeutics, CRC Oncology, and Cytokinetics. Dr Moslehi and Salem have patents pending related to the treatment of immune-related adverse events including ICI myocarditis.
Figures
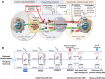
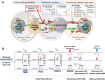
References
-
- Geraud A., Gougis P., Vozy A., et al. Clinical pharmacology and interplay of immune checkpoint agents: a yin-yang balance. Annu Rev Pharmacol Toxicol. 2021;61:85–112. - PubMed
-
- Lehmann L.H., Cautela J., Palaskas N., et al. Clinical strategy for the diagnosis and treatment of immune checkpoint inhibitor-associated myocarditis: a narrative review. JAMA Cardiol. 2021;6:1329–1337. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

