On-demand biomanufacturing through synthetic biology approach
- PMID: 36636637
- PMCID: PMC9830231
- DOI: 10.1016/j.mtbio.2022.100518
On-demand biomanufacturing through synthetic biology approach
Abstract
Biopharmaceuticals including protein therapeutics, engineered protein-based vaccines and monoclonal antibodies, are currently the mainstay products of the biotechnology industry. However, the need for specialized equipment and refrigeration during production and distribution poses challenges for the delivery of these technologies to the field and low-resource area. With the development of synthetic biology, multiple studies rewire the cell-free system or living cells to impact the portable, on-site and on-demand manufacturing of biomolecules. Here, we review these efforts and suggest future directions.
Keywords: Biomanufacturing; Cell-free system; Engineered microorganisms; On-demand; Portability; Synthetic biology.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
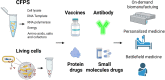

- a.
Embedding cell-free synthetic gene networks onto papers and other materials for rapid sensing and diagnoses [25].
- b.
Engineering the freeze-dried cell-free system to produce various therapeutics including antimicrobial peptides (AMPs), vaccines, combinatorial antibodies and small molecules on-site and on-demand [27].
- c.
A modular technology for in vitro conjugate vaccine expression (iVAX) in a portable and on-demand fashion [28].
- d.
Cell-free workflow for modular synthesis, assembly and discovery of multi-enzyme glycosylation pathways in vitro [37].

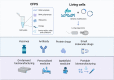
- a.
Developing microbial swarmbot (MSB) platform that integrates the multiple steps of production, disruption and separation in a concise format [49].
- b.
Engineering a temperature-responsive, shear-thinning hydrogel system to harness the bioactivity of embedded microbes for on-demand production of small molecules and peptides [50].
- c.
An integrated, benchtop and portable microfluidic device containing genetically engineered P. pastoris to generate multiple therapeutic proteins [51].
- d.
An automated desktop multi-product manufacturing system named InSCyT (Integrated Scalable Cyto-Technology) to integrate fermentation, sensing, input and output, purification and analysis automatically [52].
References
LinkOut - more resources
Full Text Sources

