Two Directing Groups Used for Metal Catalysed Meta-C-H Functionalisation Only Effect Ortho Electrophilic C-H Borylation
- PMID: 36636663
- PMCID: PMC9828458
- DOI: 10.1002/ejoc.202200901
Two Directing Groups Used for Metal Catalysed Meta-C-H Functionalisation Only Effect Ortho Electrophilic C-H Borylation
Abstract
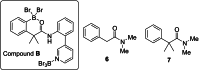
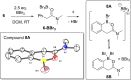
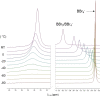
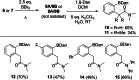
Two templates used in meta-directed C-H functionalisation under metal catalysis do not direct meta-C-H borylation under electrophilic borylation conditions. Using BCl3 only Lewis adduct formation with Lewis basic sites in the template is observed. While combining BBr3 and the template containing an amide linker only led to amide directed ortho C-H borylation, with no pyridyl directed meta borylation. The amide directed borylation is selective for the ortho borylation of the aniline derived unit in the template, with no ortho borylation of the phenylacetyl ring - which would also form a six membered boracycle - observed. In the absence of other aromatics amide directed ortho borylation on to phenylacetyl rings can be achieved. The absence of meta-borylation using two templates indicates a higher barrier to pyridyl directed meta borylation relative to amide directed ortho borylation and suggests that bespoke templates for enabling meta-directed electrophilic borylation may be required.
Keywords: Boron; Directing groups; Electrophilic substitution; Meta-C−H Functionalisation.
© 2022 The Authors. European Journal of Organic Chemistry published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- None
-
- Ros A., Fernández R., Lassaletta J. M., Chem. Soc. Rev. 2014, 43, 3229–3243; - PubMed
-
- Rej S., Ano Y., Chatani N., Chem. Rev. 2020, 120, 1788–1887; - PubMed
-
- Zhang M., Zhang Y., Jie X., Zhao H., Li G., Su W., Org. Chem. Front. 2014, 1, 843–895;
-
- Iqbal S. A., Pahl J., Yuan K., Ingleson M. J., Chem. Soc. Rev. 2020, 49, 4564–4591; - PubMed
LinkOut - more resources
Full Text Sources
