Adult T-Cell Leukemia-Lymphoma Presenting Concurrently with Myelopathy
- PMID: 36636671
- PMCID: PMC9830276
- DOI: 10.1159/000525174
Adult T-Cell Leukemia-Lymphoma Presenting Concurrently with Myelopathy
Abstract
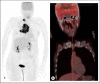
Human T-cell leukemia virus type 1 (HTLV-1) is an oncogenic retrovirus. Of the approximate ten to twenty million people currently infected worldwide, 4-9% of infected individuals develop adult T-cell leukemia/lymphoma (ATLL) or HTLV-associated myelopathy/tropical spastic paresis (HAM/TSP) in their lifetime. The current report is based on a patient who presented concurrently with CD30+ lymphoma subtype ATLL and HAM/TSP. The patient's ATLL responded to brentuximab-vedotin-based chemotherapy; however, HAM/TSP did not improve. The patient's peripheral blood mononuclear cells were cultured and injected into immunodeficient mice, and the mice developed massive organ involvement and chronic lymphocytic leukemia-subtype ATLL. This case study is novel in the findings of concurrent development of ATLL and HAM/TSP, the response to brentuximab-vedotin chemotherapy, and the use HTLV-1 helix basic zipper protein-targeted probe for RNAscope for diagnosis.
Keywords: Adult T-cell leukemia/lymphoma; Brentuximab-vedotin; HTLV-associated myelopathy/tropical spastic paresis.
Copyright © 2022 by The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

