Transthyretin Cardiac Amyloidosis Mimicking Immune Checkpoint-Induced Myocarditis in a Patient Treated with Atezolizumab and Bevacizumab for Advanced Hepatocellular Carcinoma: A Case Report
- PMID: 36636682
- PMCID: PMC9830279
- DOI: 10.1159/000526534
Transthyretin Cardiac Amyloidosis Mimicking Immune Checkpoint-Induced Myocarditis in a Patient Treated with Atezolizumab and Bevacizumab for Advanced Hepatocellular Carcinoma: A Case Report
Abstract
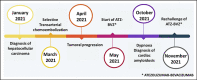
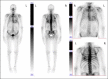
Checkpoint kinase inhibitors are increasingly used in oncology. The combination of atezolizumab and bevacizumab is currently the recommended first-line treatment for advanced hepatocellular carcinoma. Cardiac toxicities of immunotherapies are rare, but can lead to discontinuation of treatment. Transthyretin cardiac amyloidosis is a rare condition, but its incidence is probably underestimated. Its symptoms may suggest immunotherapy-induced myocarditis. When immune-mediated myocarditis is suspected, a thorough cardiac evaluation is necessary to confirm or refute the diagnosis of myocarditis and to avoid unnecessary interruption of immunotherapy. Cardiac magnetic resonance imaging may raise suspicion of transthyretin cardiac amyloidosis, the diagnosis being confirmed by technetium pyrophosphate bone scintigraphy.
Keywords: Checkpoint-kinase inhibitor; Immunotherapy; Myocarditis; Transthyretin cardiac amyloidosis.
Copyright © 2022 by The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
None of the authors declared conflicts of interest.
Figures

References
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008 Jul 24;359((4)):378–390. - PubMed
-
- Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma a randomised phase 3 non-inferiority trial. Lancet. 2018 Mar 24;391((10126)):1163–1173. - PubMed
-
- Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE) a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017 Jan 7;389((10064)):56–66. - PubMed
-
- Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2) a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019 Feb;20((2)):282–296. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

