Early intestinal microbiota changes in aged and adult mice with sepsis
- PMID: 36636721
- PMCID: PMC9831679
- DOI: 10.3389/fcimb.2022.1061444
Early intestinal microbiota changes in aged and adult mice with sepsis
Abstract
Background: The mortality rate associated with sepsis in elderly individuals is higher than that in younger individuals. The intestinal microbiota has been demonstrated to play an important role in the occurrence and development of sepsis. The purpose of this study was to investigate the differences in the intestinal microbiota between aged and adult mice with sepsis.
Methods: Thirty male C57BL mice were randomly divided into two groups: 15 in the adult group (AD group) and 15 in the age group (Age group). All the mice underwent caecal ligation and puncture to induce sepsis. Mice faeces were collected, and analysed using 16S rRNA sequencing. The liver and colon tissues were collected.
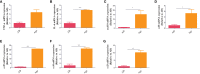
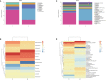
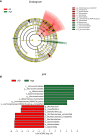
Results: There were significant differences in intestinal microbiota composition between the two groups. Compared with adult sepsis mice, the diversity of intestinal microbiota in the aged group was significantly reduced and the structure of dominant intestinal microbiota was changed. In the Age group, the microbiota associated with inflammatory factors increased, and the microbiota associated with the production of SCFAs (Ruminiclostridium, Prevotellaceae_UCG-001, Rikenella, Parabacteroides, Oscillibacter, Odoribacter, Muribaculum, Lachnoclostridium, Intestinimonas, Faecalibaculum, Anaerotruncus, Alloprevotella and Absiella) decreased. The metabolic pathways related to the microbiota also changed. Moreover, the proportion of inflammatory factors in Age group was higher than that in AD group.
Conclusion: Our results showed that there were significant differences in the abundance and structure of microbiota between aged and adult sepsis mice, Aged sepsis mice have more severe intestinal microbiota destruction and liver tissue inflammation than adult sepsis mice.
Keywords: 16S; adult mice; aged mice; intestinal microbiota; sepsis.
Copyright © 2022 Yuan, Liu, Ding, Li, Zhang, Song, Qi, Zhang, Guo and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates microbiota dysbiosis in an experimental model of sepsis.Int J Mol Med. 2019 Mar;43(3):1139-1148. doi: 10.3892/ijmm.2019.4050. Epub 2019 Jan 7. Int J Mol Med. 2019. PMID: 30628657 Free PMC article.
-
[Composition and changes of intestinal flora in septic mouse model].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021 Jan;33(1):10-16. doi: 10.3760/cma.j.cn121430-20200814-00579. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021. PMID: 33565393 Chinese.
-
A potential marker of radiation based on 16S rDNA in the rat model: Intestinal flora.PLoS One. 2023 Aug 1;18(8):e0286026. doi: 10.1371/journal.pone.0286026. eCollection 2023. PLoS One. 2023. PMID: 37527262 Free PMC article.
-
Modulation of faecal metagenome in Crohn's disease: Role of microRNAs as biomarkers.World J Gastroenterol. 2018 Dec 14;24(46):5223-5233. doi: 10.3748/wjg.v24.i46.5223. World J Gastroenterol. 2018. PMID: 30581271 Free PMC article.
-
VSL#3 can prevent ulcerative colitis-associated carcinogenesis in mice.World J Gastroenterol. 2018 Oct 7;24(37):4254-4262. doi: 10.3748/wjg.v24.i37.4254. World J Gastroenterol. 2018. PMID: 30310258 Free PMC article.
Cited by
-
Therapeutic Effects of HLA-G5 Overexpressing hAMSCs on aGVHD After Allo-HSCT: Involving in the Gut Microbiota at the Intestinal Barrier.J Inflamm Res. 2023 Aug 24;16:3669-3685. doi: 10.2147/JIR.S420747. eCollection 2023. J Inflamm Res. 2023. PMID: 37645691 Free PMC article.
-
Curcumin alleviates imiquimod-induced psoriasis-like inflammation and regulates gut microbiota of mice.Immun Inflamm Dis. 2023 Aug;11(8):e967. doi: 10.1002/iid3.967. Immun Inflamm Dis. 2023. PMID: 37647442 Free PMC article.
-
Ozone controls the metabolism of tryptophan protecting against sepsis-induced intestinal damage by activating aryl hydrocarbon receptor.World J Gastroenterol. 2025 May 7;31(17):105411. doi: 10.3748/wjg.v31.i17.105411. World J Gastroenterol. 2025. PMID: 40521273 Free PMC article.
-
Vitamin K2 supplementation improves impaired glycemic homeostasis and insulin sensitivity for type 2 diabetes through gut microbiome and fecal metabolites.BMC Med. 2023 May 5;21(1):174. doi: 10.1186/s12916-023-02880-0. BMC Med. 2023. PMID: 37147641 Free PMC article.
-
Gold nanoparticles exhibit anti-osteoarthritic effects via modulating interaction of the "microbiota-gut-joint" axis.J Nanobiotechnology. 2024 Apr 8;22(1):157. doi: 10.1186/s12951-024-02447-y. J Nanobiotechnology. 2024. PMID: 38589904 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

