Taste adaptations associated with host specialization in the specialist Drosophila sechellia
- PMID: 36637369
- PMCID: PMC10088416
- DOI: 10.1242/jeb.244641
Taste adaptations associated with host specialization in the specialist Drosophila sechellia
Abstract
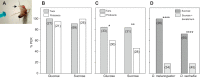
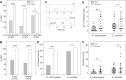
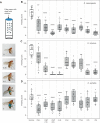
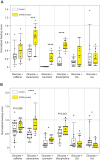
Chemosensory-driven host plant specialization is a major force mediating insect ecological adaptation and speciation. Drosophila sechellia, a species endemic to the Seychelles islands, feeds and oviposits on Morinda citrifolia almost exclusively. This fruit is harmless to D. sechellia but toxic to other Drosophilidae, including the closely related generalists D. simulans and D. melanogaster, because of its high content of fatty acids. While several olfactory adaptations mediating D. sechellia's preference for its host have been uncovered, the role of taste has been much less examined. We found that D. sechellia has reduced taste and feeding aversion to bitter compounds and host fatty acids that are aversive to D. melanogaster and D. simulans. The loss of aversion to canavanine, coumarin and fatty acids arose in the D. sechellia lineage, as its sister species D. simulans showed responses akin to those of D. melanogaster. Drosophila sechellia has increased taste and feeding responses towards M. citrifolia. These results are in line with D. sechellia's loss of genes that encode bitter gustatory receptors (GRs) in D. melanogaster. We found that two GR genes which are lost in D. sechellia, GR39a.a and GR28b.a, influence the reduction of aversive responses to some bitter compounds. Also, D. sechellia has increased appetite for a prominent host fatty acid compound that is toxic to its relatives. Our results support the hypothesis that changes in the taste system, specifically a reduction of sensitivity to bitter compounds that deter generalist ancestors, contribute to the specialization of D. sechellia for its host.
Keywords: Drosophila sechellia; Behavior; Bitter receptor; Chemosensation; Ecological adaptation; Gustatory receptors; Host plant specialization; Noni.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


Similar articles
-
Evolution of fatty acid taste in drosophilids.Cell Rep. 2023 Oct 31;42(10):113297. doi: 10.1016/j.celrep.2023.113297. Cell Rep. 2023. PMID: 37864792 Free PMC article.
-
Odorant-binding proteins OBP57d and OBP57e affect taste perception and host-plant preference in Drosophila sechellia.PLoS Biol. 2007 May;5(5):e118. doi: 10.1371/journal.pbio.0050118. PLoS Biol. 2007. PMID: 17456006 Free PMC article.
-
Genetic changes accompanying the evolution of host specialization in Drosophila sechellia.Genetics. 2009 Feb;181(2):721-36. doi: 10.1534/genetics.108.093419. Epub 2008 Nov 24. Genetics. 2009. PMID: 19033155 Free PMC article.
-
The genetics of adaptation in Drosophila sechellia.Genetica. 2005 Feb;123(1-2):137-45. doi: 10.1007/s10709-004-2728-6. Genetica. 2005. PMID: 15881686 Review.
-
Drosophila sechellia: A Genetic Model for Behavioral Evolution and Neuroecology.Annu Rev Genet. 2021 Nov 23;55:527-554. doi: 10.1146/annurev-genet-071719-020719. Epub 2021 Sep 16. Annu Rev Genet. 2021. PMID: 34530638 Review.
Cited by
-
Comparative single-cell transcriptomic atlases of drosophilid brains suggest glial evolution during ecological adaptation.PLoS Biol. 2025 Apr 29;23(4):e3003120. doi: 10.1371/journal.pbio.3003120. eCollection 2025 Apr. PLoS Biol. 2025. PMID: 40299832 Free PMC article.
-
Evolution of chemosensory and detoxification gene families across herbivorous Drosophilidae.G3 (Bethesda). 2023 Aug 9;13(8):jkad133. doi: 10.1093/g3journal/jkad133. G3 (Bethesda). 2023. PMID: 37317982 Free PMC article.
-
Evolution of fatty acid taste in drosophilids.Cell Rep. 2023 Oct 31;42(10):113297. doi: 10.1016/j.celrep.2023.113297. Cell Rep. 2023. PMID: 37864792 Free PMC article.
-
Evolution of chemosensory and detoxification gene families across herbivorous Drosophilidae.bioRxiv [Preprint]. 2023 Mar 16:2023.03.16.532987. doi: 10.1101/2023.03.16.532987. bioRxiv. 2023. Update in: G3 (Bethesda). 2023 Aug 9;13(8):jkad133. doi: 10.1093/g3journal/jkad133. PMID: 36993186 Free PMC article. Updated. Preprint.
References
-
- Abou Assi, R., Darwis, Y., Abdulbaqi, I. M., khan, A. A., Vuanghao, L. and Laghari, M. H. (2017). Morinda citrifolia (Noni): a comprehensive review on its industrial uses, pharmacological activities, and clinical trials. Arab. J. Chem. 10, 691-707.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

