Myelination generates aberrant ultrastructure that is resolved by microglia
- PMID: 36637807
- PMCID: PMC9856851
- DOI: 10.1083/jcb.202204010
Myelination generates aberrant ultrastructure that is resolved by microglia
Abstract
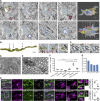
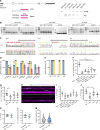
To enable rapid propagation of action potentials, axons are ensheathed by myelin, a multilayered insulating membrane formed by oligodendrocytes. Most of the myelin is generated early in development, resulting in the generation of long-lasting stable membrane structures. Here, we explored structural and dynamic changes in central nervous system myelin during development. To achieve this, we performed an ultrastructural analysis of mouse optic nerves by serial block face scanning electron microscopy (SBF-SEM) and confocal time-lapse imaging in the zebrafish spinal cord. We found that myelin undergoes extensive ultrastructural changes during early postnatal development. Myelin degeneration profiles were engulfed and phagocytosed by microglia using exposed phosphatidylserine as one "eat me" signal. In contrast, retractions of entire myelin sheaths occurred independently of microglia and involved uptake of myelin by the oligodendrocyte itself. Our findings show that the generation of myelin early in development is an inaccurate process associated with aberrant ultrastructural features that require substantial refinement.
© 2023 Djannatian et al.
Conflict of interest statement
Disclosures: The authors declare no competing financial interests.
Figures







References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

