SGLT2 inhibitors mitigate kidney tubular metabolic and mTORC1 perturbations in youth-onset type 2 diabetes
- PMID: 36637914
- PMCID: PMC9974101
- DOI: 10.1172/JCI164486
SGLT2 inhibitors mitigate kidney tubular metabolic and mTORC1 perturbations in youth-onset type 2 diabetes
Abstract
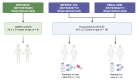
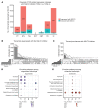
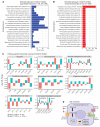
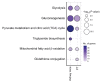
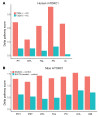
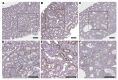
The molecular mechanisms of sodium-glucose cotransporter-2 (SGLT2) inhibitors (SGLT2i) remain incompletely understood. Single-cell RNA sequencing and morphometric data were collected from research kidney biopsies donated by young persons with type 2 diabetes (T2D), aged 12 to 21 years, and healthy controls (HCs). Participants with T2D were obese and had higher estimated glomerular filtration rates and mesangial and glomerular volumes than HCs. Ten T2D participants had been prescribed SGLT2i (T2Di[+]) and 6 not (T2Di[-]). Transcriptional profiles showed SGLT2 expression exclusively in the proximal tubular (PT) cluster with highest expression in T2Di(-) patients. However, transcriptional alterations with SGLT2i treatment were seen across nephron segments, particularly in the distal nephron. SGLT2i treatment was associated with suppression of transcripts in the glycolysis, gluconeogenesis, and tricarboxylic acid cycle pathways in PT, but had the opposite effect in thick ascending limb. Transcripts in the energy-sensitive mTORC1-signaling pathway returned toward HC levels in all tubular segments in T2Di(+), consistent with a diabetes mouse model treated with SGLT2i. Decreased levels of phosphorylated S6 protein in proximal and distal tubules in T2Di(+) patients confirmed changes in mTORC1 pathway activity. We propose that SGLT2i treatment benefits the kidneys by mitigating diabetes-induced metabolic perturbations via suppression of mTORC1 signaling in kidney tubules.
Keywords: Chronic kidney disease; Diabetes; Metabolism; Nephrology; Transcription.
Figures


Comment in
-
SGLT2 inhibitors attenuate alterations of kidney metabolic pathways in youth-onset T2DM.Nat Rev Nephrol. 2023 Apr;19(4):213. doi: 10.1038/s41581-023-00698-w. Nat Rev Nephrol. 2023. PMID: 36849740 No abstract available.
References
-
- Bjornstad P, et al. Elevated serum uric acid is associated with greater risk for hypertension and diabetic kidney diseases in obese adolescents with type 2 diabetes: an observational analysis from the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study. Diabetes Care. 2019;42(6):1120–1128. doi: 10.2337/dc18-2147. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 DK124449/DK/NIDDK NIH HHS/United States
- R01 DK124709/DK/NIDDK NIH HHS/United States
- R01 GM145631/GM/NIGMS NIH HHS/United States
- P30 DK116073/DK/NIDDK NIH HHS/United States
- R01 DK132399/DK/NIDDK NIH HHS/United States
- K23 DK116720/DK/NIDDK NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- P30 DK081943/DK/NIDDK NIH HHS/United States
- R01 DK129211/DK/NIDDK NIH HHS/United States
- U24 DK114886/DK/NIDDK NIH HHS/United States
- R01 HL165433/HL/NHLBI NIH HHS/United States
- K23 DK125529/DK/NIDDK NIH HHS/United States
- R01 DK100449/DK/NIDDK NIH HHS/United States
- R21 DK129720/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

