Increased length-dependent activation of human engineered heart tissue after chronic α1A-adrenergic agonist treatment: testing a novel heart failure therapy
- PMID: 36637971
- PMCID: PMC9886349
- DOI: 10.1152/ajpheart.00279.2022
Increased length-dependent activation of human engineered heart tissue after chronic α1A-adrenergic agonist treatment: testing a novel heart failure therapy
Abstract
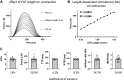
Chronic stimulation of cardiac α1A-adrenergic receptors (α1A-ARs) improves symptoms in multiple preclinical models of heart failure. However, the translational significance remains unclear. Human engineered heart tissues (EHTs) provide a means of quantifying the effects of chronic α1A-AR stimulation on human cardiomyocyte physiology. EHTs were created from thin slices of decellularized pig myocardium seeded with human induced pluripotent stem cell (iPSC)-derived cardiomyocytes and fibroblasts. With a paired experimental design, EHTs were cultured for 3 wk, mechanically tested, cultured again for 2 wk with α1A-AR agonist A61603 (10 nM) or vehicle control, and retested after drug washout for 24 h. Separate control experiments determined the effects of EHT age (3-5 wk) or repeat mechanical testing. We found that chronic A61603 treatment caused a 25% increase of length-dependent activation (LDA) of contraction compared with vehicle treatment (n = 7/group, P = 0.035). EHT force was not increased after chronic A61603 treatment. However, after vehicle treatment, EHT force was increased by 35% relative to baseline testing (n = 7/group, P = 0.022), suggesting EHT maturation. Control experiments suggested that increased EHT force resulted from repeat mechanical testing, not from EHT aging. RNA-seq analysis confirmed that the α1A-AR is expressed in human EHTs and found chronic A61603 treatment affected gene expression in biological pathways known to be activated by α1A-ARs, including the MAP kinase signaling pathway. In conclusion, increased LDA in human EHT after chronic A61603 treatment raises the possibility that chronic stimulation of the α1A-AR might be beneficial for increasing LDA in human myocardium and might be beneficial for treating human heart failure by restoring LDA.NEW & NOTEWORTHY Chronic stimulation of α1A-adrenergic receptors (α1A-ARs) is known to mediate therapeutic effects in animal heart failure models. To investigate the effects of chronic α1A-AR stimulation in human cardiomyocytes, we tested engineered heart tissue (EHT) created with iPSC-derived cardiomyocytes. RNA-seq analysis confirmed human EHT expressed α1A-ARs. Chronic (2 wk) α1A-AR stimulation with A61603 (10 nM) increased length-dependent activation (LDA) of contraction. Chronic α1A-AR stimulation might be beneficial for treating human heart failure by restoring LDA.
Keywords: engineered heart tissue; heart failure; human; induced pluripotent stem cell; α1A-adrenergic receptor.
Conflict of interest statement
Propria, LLC, has a financial interest in MyoPod products/services presented herein. S.G.C. is the founder of, holds equity in, and has received consulting fees from Propria, LLC. The experiments reported in the submitted manuscript were performed at Propria LLC. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

