State and Future Science of Opioids and Potential of Biased-ligand Technology in the Management of Acute Pain After Burn Injury
- PMID: 36638083
- PMCID: PMC10152994
- DOI: 10.1093/jbcr/irad004
State and Future Science of Opioids and Potential of Biased-ligand Technology in the Management of Acute Pain After Burn Injury
Abstract
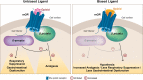
Pain associated with severe burn injury is one of the most intense and clinically challenging to manage, as the metabolic imbalances associated with the inflammation caused by the injury and treatment interventions (e.g., dressing changes and debridement, excision, and grafting) can further worsen the pain. In the pharmacologic management of a complex, hospitalized patient with burn injuries, opioid therapy remains an efficacious mainstay of treatment. However, the complex nature of pain, injury characteristics, and common demographics after burn injury place patients at high risk of opioid-related adverse events. Thus, guidelines recommend that decisions about choice of opioid be based on physiology, pharmacology, and physician experience, in addition to individualizing initial treatment with subsequent continual adjustments throughout care. Although substantial progress has been made in pain management strategies with utilization of nonopioid medications and nonpharmacologic adjuncts to opioid pharmacotherapy, there is still a need to evaluate new therapies, as an optimal regimen still lacks significant evidential support. Herein, we review the actions of opioids at the cellular level, contributing to both nociception and opioid-related adverse events. We also discuss the most recently approved intravenously administered opioid, oliceridine, developed utilizing biased ligand technology, including a summary of its clinical efficacy and safety in the management of severe acute pain. While oliceridine has been evaluated for the management of moderate-to-severe acute pain, the large phase 3 studies did not include patients with burn injuries. However, potential implications and future study direction for pain associated with burn injury are discussed.
Keywords: Adverse Drug Event; Analgesics; Burns; Opioid; Pain.
© The Author(s) 2023. Published by Oxford University Press on behalf of the American Burn Association.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

