prM-reactive antibodies reveal a role for partially mature virions in dengue virus pathogenesis
- PMID: 36638211
- PMCID: PMC9933121
- DOI: 10.1073/pnas.2218899120
prM-reactive antibodies reveal a role for partially mature virions in dengue virus pathogenesis
Abstract
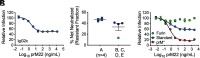
Cleavage of the flavivirus premembrane (prM) structural protein during maturation can be inefficient. The contribution of partially mature flavivirus virions that retain uncleaved prM to pathogenesis during primary infection is unknown. To investigate this question, we characterized the functional properties of newly-generated dengue virus (DENV) prM-reactive monoclonal antibodies (mAbs) in vitro and using a mouse model of DENV disease. Anti-prM mAbs neutralized DENV infection in a virion maturation state-dependent manner. Alanine scanning mutagenesis and cryoelectron microscopy of anti-prM mAbs in complex with immature DENV defined two modes of attachment to a single antigenic site. In vivo, passive transfer of intact anti-prM mAbs resulted in an antibody-dependent enhancement of disease. However, protection against DENV-induced lethality was observed when the transferred mAbs were genetically modified to inhibit their ability to interact with Fcγ receptors. These data establish that in addition to mature forms of the virus, partially mature infectious prM+ virions can also contribute to pathogenesis during primary DENV infections.
Keywords: antibody-dependent enhancement; dengue virus; prM antibody; virion maturation.
Conflict of interest statement
The authors have organizational affiliations to disclose, M.S.D. is a consultant for Inbios, Vir Biotechnology, Moderna, Novavax, Senda Biosciences and Immunome. Yes, the authors have research support to disclose. The Diamond laboratory has received unrelated funding support in sponsored research agreements from Moderna, Vir Biotechnology, and Emergent BioSolutions.
Figures





References
-
- Gould E. A., Solomon T., Pathogenic flaviviruses. Lancet 371, 500–509 (2008). - PubMed
-
- World Health Organization, Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition, World Health Organization, Ed., (World Health Organization, Geneva, 2009). - PubMed
-
- Guzman M. G., Alvarez M., Halstead S. B., Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: An historical perspective and role of antibody-dependent enhancement of infection. Arch. Virol. 158, 1445–1459 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

