Loss of epigenetic information as a cause of mammalian aging
- PMID: 36638792
- PMCID: PMC10166133
- DOI: 10.1016/j.cell.2022.12.027
Loss of epigenetic information as a cause of mammalian aging
Erratum in
-
Loss of epigenetic information as a cause of mammalian aging.Cell. 2024 Feb 29;187(5):1312-1313. doi: 10.1016/j.cell.2024.01.049. Cell. 2024. PMID: 38428398 No abstract available.
Abstract
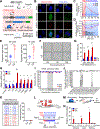
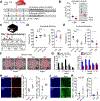
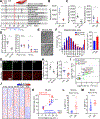
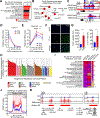
All living things experience an increase in entropy, manifested as a loss of genetic and epigenetic information. In yeast, epigenetic information is lost over time due to the relocalization of chromatin-modifying proteins to DNA breaks, causing cells to lose their identity, a hallmark of yeast aging. Using a system called "ICE" (inducible changes to the epigenome), we find that the act of faithful DNA repair advances aging at physiological, cognitive, and molecular levels, including erosion of the epigenetic landscape, cellular exdifferentiation, senescence, and advancement of the DNA methylation clock, which can be reversed by OSK-mediated rejuvenation. These data are consistent with the information theory of aging, which states that a loss of epigenetic information is a reversible cause of aging.
Keywords: DNA damage; RCM; aging; chromatin; epigenetic clock; epigenetic reprogramming; relocalization of chromatin modifier; senescence.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.A.S. is a consultant, inventor, board member, and in some cases an investor in Life Biosciences (developing reprogramming medicines), InsideTracker, Zymo, EdenRoc Sciences/Cantata/Dovetail/Metrobiotech, Caudalie, Galilei, Immetas, Animal Biosciences, Tally Health, and more. See https://sinclair.hms.harvard.edu/david-sinclairs-affiliations. E.M.M., M. Blanchette, and M. Bhakta are employees of Catata Bio/Dovetail. Y.C.C., W.G., and X.Y. are employees of Zymo Research. A.J.W. advises Kate Therapeutics and Frequency Therapeutics and is a co-founder, adviser, and equity holder of Elevian, which sponsors Wagers Lab research. L.S. was an employee of Vium. Y.R.L. and L.A.R. are equity owners of Life Biosciences. M.S.B. and D.L.V. advise EdenRoc Sciences. A patent application was filed on the reprogramming methods.
Figures



Comment in
-
Putting aging on ICE.Cell Metab. 2023 Mar 7;35(3):383-385. doi: 10.1016/j.cmet.2023.02.012. Cell Metab. 2023. PMID: 36889279 Free PMC article.
-
The loss of epigenetic information: not only consequences but a cause of mammalian aging.Signal Transduct Target Ther. 2023 Mar 27;8(1):140. doi: 10.1038/s41392-023-01412-9. Signal Transduct Target Ther. 2023. PMID: 36973241 Free PMC article. No abstract available.
References
-
- Baruch K, Deczkowska A, David E, Castellano JM, Miller O, Kertser A, Berkutzki T, Barnett-Itzhaki Z, Bezalel D, Wyss-Coray T, et al. (2014). Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science (New York, NY) 346, 89–93. 10.1126/science.1252945. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY019703/EY/NEI NIH HHS/United States
- F99 AG073499/AG/NIA NIH HHS/United States
- K99 AG068303/AG/NIA NIH HHS/United States
- K99 AG055683/AG/NIA NIH HHS/United States
- R37 AG028730/AG/NIA NIH HHS/United States
- P01 AG051449/AG/NIA NIH HHS/United States
- R00 AG070102/AG/NIA NIH HHS/United States
- P30 EY003790/EY/NEI NIH HHS/United States
- R01 AG019719/AG/NIA NIH HHS/United States
- R01 DK056799/DK/NIDDK NIH HHS/United States
- R01 AG065403/AG/NIA NIH HHS/United States
- R01 DK097598/DK/NIDDK NIH HHS/United States
- R01 AG064223/AG/NIA NIH HHS/United States
- P01 AG031862/AG/NIA NIH HHS/United States
- R01 AG028730/AG/NIA NIH HHS/United States
- R01 AG016694/AG/NIA NIH HHS/United States
- R00 AG055683/AG/NIA NIH HHS/United States
- T32 HL007118/HL/NHLBI NIH HHS/United States
- K99 AG070102/AG/NIA NIH HHS/United States
- T32 AG023480/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

