Interleukin-13 and its receptor are synaptic proteins involved in plasticity and neuroprotection
- PMID: 36639371
- PMCID: PMC9839781
- DOI: 10.1038/s41467-023-35806-8
Interleukin-13 and its receptor are synaptic proteins involved in plasticity and neuroprotection
Abstract
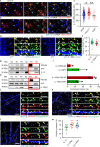
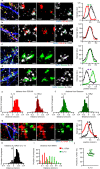
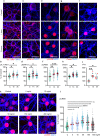
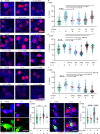
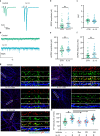
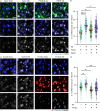
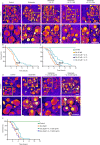
Immune system molecules are expressed by neurons, yet their functions are often unknown. We have identified IL-13 and its receptor IL-13Ra1 as neuronal, synaptic proteins in mouse, rat, and human brains, whose engagement upregulates the phosphorylation of NMDAR and AMPAR subunits and, in turn, increases synaptic activity and CREB-mediated transcription. We demonstrate that increased IL-13 is a hallmark of traumatic brain injury (TBI) in male mice as well as in two distinct cohorts of human patients. We also provide evidence that IL-13 upregulation protects neurons from excitotoxic death. We show IL-13 upregulation occurring in several cohorts of human brain samples and in cerebrospinal fluid (CSF). Thus, IL-13 is a physiological modulator of synaptic physiology of neuronal origin, with implications for the establishment of synaptic plasticity and the survival of neurons under injury conditions. Furthermore, we suggest that the neuroprotection afforded through the upregulation of IL-13 represents an entry point for interventions in the pathophysiology of TBI.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

