High-throughput analysis of the transcriptional patterns of sexual genes in malaria
- PMID: 36639683
- PMCID: PMC9838061
- DOI: 10.1186/s13071-022-05624-w
High-throughput analysis of the transcriptional patterns of sexual genes in malaria
Abstract
Background: Plasmodium falciparum (Pf) is the leading protozoan causing malaria, the most devastating parasitic disease. To ensure transmission, a small subset of Pf parasites differentiate into the sexual forms (gametocytes). Since the abundance of these essential parasitic forms is extremely low within the human host, little is currently known about the molecular regulation of their sexual differentiation, highlighting the need to develop tools to investigate Pf gene expression during this fundamental mechanism.
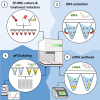
Methods: We developed a high-throughput quantitative Reverse-Transcription PCR (RT-qPCR) platform to robustly monitor Pf transcriptional patterns, in particular, systematically profiling the transcriptional pattern of a large panel of gametocyte-related genes (GRG). Initially, we evaluated the technical performance of the systematic RT-qPCR platform to ensure it complies with the accepted quality standards for: (i) RNA extraction, (ii) cDNA synthesis and (iii) evaluation of gene expression through RT-qPCR. We then used this approach to monitor alterations in gene expression of a panel of GRG upon treatment with gametocytogenesis regulators.
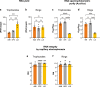
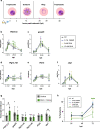
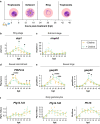
Results: We thoroughly elucidated GRG expression profiles under treatment with the antimalarial drug dihydroartemisinin (DHA) or the metabolite choline over the course of a Pf blood cycle (48 h). We demonstrate that both significantly alter the expression pattern of PfAP2-G, the gametocytogenesis master regulator. However, they also markedly modify the developmental rate of the parasites and thus might bias the mRNA expression. Additionally, we screened the effect of the metabolites lactate and kynurenic acid, abundant in severe malaria, as potential regulators of gametocytogenesis.
Conclusions: Our data demonstrate that the high-throughput RT-qPCR method enables studying the immediate transcriptional response initiating gametocytogenesis of the parasites from a very low volume of malaria-infected RBC samples. The obtained data expand the current knowledge of the initial alterations in mRNA profiles of GRG upon treatment with reported regulators. In addition, using this method emphasizes that asexual parasite stage composition is a crucial element that must be considered when interpreting changes in GRG expression by RT-qPCR, specifically when screening for novel compounds that could regulate Pf sexual differentiation.
Keywords: Automatization; Gametocyte; Gametocytogenesis; Gene expression; Malaria; Plasmodium falciparum; RT-qPCR.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- World malaria report 2022. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO.
-
- World malaria report 2020: 20 years of global progress and challenges. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

