Evolution of treatment patterns and survival outcomes in patients with advanced non-small cell lung cancer treated at Frankfurt University Hospital in 2012-2018
- PMID: 36639770
- PMCID: PMC9838033
- DOI: 10.1186/s12890-022-02288-1
Evolution of treatment patterns and survival outcomes in patients with advanced non-small cell lung cancer treated at Frankfurt University Hospital in 2012-2018
Abstract
Background: Immune checkpoint inhibitors (ICIs) have improved outcomes for patients with advanced non-small cell lung cancer (NSCLC) versus chemotherapy in clinical trials. In Germany, ICIs have been used clinically since 2015 for patients with advanced/metastatic NSCLC without epidermal growth factor receptor (EGFR)/anaplastic lymphoma kinase (ALK) aberrations. As part of I-O Optimise, a multinational research program utilizing real-world data on thoracic malignancies, we describe real-world treatment patterns and survival following reimbursement of ICIs for advanced NSCLC in Germany.
Methods: This retrospective cohort study included patients with locally advanced/metastatic NSCLC without known EGFR/ALK aberrations who received a first line of therapy at Frankfurt University Hospital between January 2012 and December 2018, with follow-up to December 2019 or death, whichever occurred first. Using electronic medical records, treatment patterns and survival outcomes were described by histology (squamous cell [SQ]; non-squamous cell [NSQ]/other) and time period (pre- and post-ICI approval).
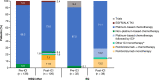
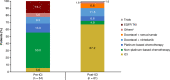
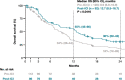
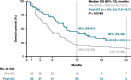
Results: Among eligible patients who started first-line treatment, 136 (pre-ICI) and 126 (post-ICI) had NSQ/other histology, and 32 (pre-ICI) and 38 (post-ICI) had SQ histology. Use of an ICI in the NSQ/other cohort increased from 5.9% (all second- or third-line) in the pre-ICI period to 57.1% (22.2% in first-line, including 13.5% as monotherapy and 8.7% combined with chemotherapy) in the post-ICI period. This was paralleled by a significant (P < 0.0001) prolongation of median (95% CI) OS from 9.4 (7.1-11.1) to 14.8 (12.7-20.5) months between the pre-ICI and post-ICI periods. A similar increase in the uptake of ICI was observed for the SQ cohort (from 3.1% pre-ICI [fourth-line] to 52.6% post-ICI [28.9% as first-line, including 15.8% as monotherapy and 13.2% combined with chemotherapy]); however, analysis of survival outcomes was limited by small group sizes.
Conclusion: These real-world data complement clinical trial evidence on the effectiveness of ICIs in patients with advanced NSCLC and NSQ/other histology in Germany.
Keywords: Immune checkpoint inhibitors; Immunotherapy; Non-small cell lung cancer; Overall survival; Real-world evidence.
© 2023. The Author(s).
Conflict of interest statement
AW reports no conflicts of interest. JAS reports personal fees from Boehringer Ingelheim, AstraZeneca, Roche, Bristol Myers Squibb, Amgen, LEO Pharma, Novartis, and Takeda outside of the submitted work. SS reports no conflicts of interest. NN, AC, HU, and RM are employees of IQVIA. DW, RC, MJD, and JRP are employees of Bristol Myers Squibb; RC and JRP also report stock ownership in Bristol Myers Squibb. LL is an employee of Epi-Fit and was contracted (paid) as a consultant by Bristol Myers Squibb to support the I-O Optimise initiative. GR reports personal fees from AstraZeneca, Berlin Chemie, Bristol Myers Squibb, Boehringer Ingelheim, Chiesi, Essex Pharma, Grifols, GSK, Insmed, MSD, Roche, Solvay, Takeda, Novartis, Pfizer, and Vertex for consultancy during advisory board meetings and personal fees from AstraZeneca, Berlin Chemie, Bristol Myers Squibb, Boehringer Ingelheim, Chiesi, Essex Pharma, Grifols, GSK, Insmed, MSD, Roche, Solvay, Takeda, Novartis, Pfizer, and Vertex for lectures including service on speakers’ bureaus.
Figures

Similar articles
-
Real-world treatment patterns and survival outcomes for advanced non-small cell lung cancer in the pre-immunotherapy era in Portugal: a retrospective analysis from the I-O Optimise initiative.BMC Pulm Med. 2020 Sep 10;20(1):240. doi: 10.1186/s12890-020-01270-z. BMC Pulm Med. 2020. PMID: 32912174 Free PMC article.
-
Long-term real-world outcomes of first-line immunotherapy in non-small cell lung cancer - a population-based cohort study in Sweden.Acta Oncol. 2025 Mar 17;64:415-422. doi: 10.2340/1651-226X.2025.42746. Acta Oncol. 2025. PMID: 40094477 Free PMC article.
-
Trends in treatment patterns and survival outcomes in advanced non-small cell lung cancer: a Canadian population-based real-world analysis.BMC Cancer. 2022 Mar 10;22(1):255. doi: 10.1186/s12885-022-09342-5. BMC Cancer. 2022. PMID: 35264135 Free PMC article.
-
Effect of histology on the efficacy of immune checkpoint inhibitors in advanced non-small cell lung cancer: A systematic review and meta-analysis.Front Oncol. 2022 Nov 10;12:968517. doi: 10.3389/fonc.2022.968517. eCollection 2022. Front Oncol. 2022. PMID: 36439448 Free PMC article.
-
Efficacy and safety of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer (NSCLC): a systematic literature review.Oncoimmunology. 2020 Jun 16;9(1):1774314. doi: 10.1080/2162402X.2020.1774314. Oncoimmunology. 2020. PMID: 32923134 Free PMC article.
Cited by
-
Association of systemic therapy with survival among adults with advanced non-small cell lung cancer.Transl Lung Cancer Res. 2025 Jan 24;14(1):176-193. doi: 10.21037/tlcr-24-749. Epub 2025 Jan 22. Transl Lung Cancer Res. 2025. PMID: 39958214 Free PMC article.
-
Real-world treatment patterns and outcomes for patients with non-metastatic non-small cell lung cancer: retrospective analyses in Canada, England, and Germany.BMC Pulm Med. 2025 May 27;25(1):265. doi: 10.1186/s12890-025-03715-9. BMC Pulm Med. 2025. PMID: 40426148 Free PMC article.
-
Antibiotic treatment and survival in non-small cell lung cancer patients receiving immunotherapy: a systematic review and meta-analysis.Transl Lung Cancer Res. 2023 Dec 26;12(12):2427-2439. doi: 10.21037/tlcr-23-597. Epub 2023 Dec 22. Transl Lung Cancer Res. 2023. PMID: 38205205 Free PMC article.
-
Advancing real-world research in thoracic malignancies: learnings from the international I-O Optimise initiative.Future Oncol. 2025 Mar;21(7):867-878. doi: 10.1080/14796694.2025.2466416. Epub 2025 Feb 25. Future Oncol. 2025. PMID: 39996596 Free PMC article. Review.
-
Shall We Screen Lung Cancer with Volume Computed Tomography in Austria? A Cost-Effectiveness Modelling Study.Cancers (Basel). 2024 Jul 23;16(15):2623. doi: 10.3390/cancers16152623. Cancers (Basel). 2024. PMID: 39123350 Free PMC article.
References
-
- International Agency for Research on Cancer. GLOBOCAN 2020. Germany. https://gco.iarc.fr/today/data/factsheets/populations/276-germany-fact-s.... Accessed 27 July 2022.
-
- Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–237. doi: 10.1093/annonc/mdy275. - DOI - PubMed
-
- National Institutes of Health, National Cancer Institute. SEER Program - Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed 27 July 2022.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

