Expression of a Form of Cerebellar Motor Memory Requires Learned Alterations to the Activity of Inhibitory Molecular Layer Interneurons
- PMID: 36639897
- PMCID: PMC9888511
- DOI: 10.1523/JNEUROSCI.0731-22.2022
Expression of a Form of Cerebellar Motor Memory Requires Learned Alterations to the Activity of Inhibitory Molecular Layer Interneurons
Abstract
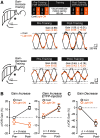
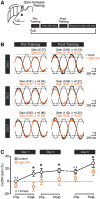
Procedural memories formed in the cerebellum in response to motor errors depend on changes to Purkinje cell (PC) spiking patterns that correct movement when the erroneous context is repeated. Because molecular layer interneurons (MLIs) inhibit PCs, learning-induced changes to MLI output may participate in reshaping PC spiking patterns. However, it remains unclear whether error-driven learning alters MLI activity and whether such changes are necessary for the memory engram. We addressed this knowledge gap by measuring and manipulating MLI activity in the flocculus of both sexes of mice before and after vestibulo-ocular reflex (VOR) adaptation. We found that MLIs are activated during vestibular stimuli and that their population response exhibits a phase shift after the instantiation of gain-increase VOR adaptation, a type of error-driven learning thought to require climbing-fiber-mediated instructive signaling. Although acute optogenetic suppression of MLI activity did not affect baseline VOR performance, it negated the expression of gain-increase learning, demonstrating a specific role of MLI activity changes in motor memory expression. This effect was transitory; after a multiday consolidation period, the expression of VOR gain-increase learning was no longer sensitive to MLI activity suppression. Together, our results indicate that error-driven alteration of MLI activity is necessary for labile, climbing-fiber-induced motor memory expression.SIGNIFICANCE STATEMENT In the cerebellum, motor learning induces an associative memory of the sensorimotor context of an erroneous movement that, when recalled, results in a new pattern of output that improves subsequent trials of performance. Our study shows that error-driven motor learning induces changes to the activity pattern of cerebellar molecular layer interneurons (MLIs) and that this new pattern of activity is required to express the corrective motor memory.
Keywords: Purkinje cells; learning; memory; plasticity.
Copyright © 2023 the authors.
Figures



Similar articles
-
Computational Theory Underlying Acute Vestibulo-ocular Reflex Motor Learning with Cerebellar Long-Term Depression and Long-Term Potentiation.Cerebellum. 2017 Aug;16(4):827-839. doi: 10.1007/s12311-017-0857-6. Cerebellum. 2017. PMID: 28444617
-
Molecular layer disinhibition unlocks climbing-fiber-instructed motor learning in the cerebellum.bioRxiv [Preprint]. 2023 Aug 4:2023.08.04.552059. doi: 10.1101/2023.08.04.552059. bioRxiv. 2023. PMID: 38654827 Free PMC article. Preprint.
-
Graded Control of Climbing-Fiber-Mediated Plasticity and Learning by Inhibition in the Cerebellum.Neuron. 2018 Sep 5;99(5):999-1015.e6. doi: 10.1016/j.neuron.2018.07.024. Epub 2018 Aug 16. Neuron. 2018. PMID: 30122378 Free PMC article.
-
Cerebellum-dependent learning: the role of multiple plasticity mechanisms.Annu Rev Neurosci. 2004;27:581-609. doi: 10.1146/annurev.neuro.27.070203.144238. Annu Rev Neurosci. 2004. PMID: 15217344 Review.
-
Motor learning in the VOR: the cerebellar component.Exp Brain Res. 2011 May;210(3-4):451-63. doi: 10.1007/s00221-011-2589-z. Epub 2011 Feb 19. Exp Brain Res. 2011. PMID: 21336828 Review.
Cited by
-
Cerebellar circuit computations for predictive motor control.Nat Rev Neurosci. 2025 Sep;26(9):538-553. doi: 10.1038/s41583-025-00936-z. Epub 2025 Jun 16. Nat Rev Neurosci. 2025. PMID: 40523942 Review.
-
Ethanol's impact on the brain: a neurobiological perspective on the mechanisms of memory impairment.Mol Biol Rep. 2024 Jun 25;51(1):782. doi: 10.1007/s11033-024-09748-3. Mol Biol Rep. 2024. PMID: 38918289 Review.
-
A normative principle governing memory transfer in cerebellar motor learning.Nat Commun. 2025 Jul 1;16(1):5479. doi: 10.1038/s41467-025-60511-z. Nat Commun. 2025. PMID: 40595477 Free PMC article.
-
Impaired cerebellar plasticity hypersensitizes sensory reflexes in SCN2A-associated ASD.Neuron. 2024 May 1;112(9):1444-1455.e5. doi: 10.1016/j.neuron.2024.01.029. Epub 2024 Feb 26. Neuron. 2024. PMID: 38412857 Free PMC article.
-
Kit Ligand and Kit receptor tyrosine kinase sustain synaptic inhibition of Purkinje cells.Elife. 2024 Mar 27;12:RP89792. doi: 10.7554/eLife.89792. Elife. 2024. PMID: 38536959 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
