Comparative evaluation of biased agonists Sarcosine1 , d-Alanine8 -Angiotensin (Ang) II (SD Ang II) and Sarcosine1 , Isoleucine8 -Ang II (SI Ang II) and their radioiodinated congeners binding to rat liver membrane AT1 receptors
- PMID: 36639940
- PMCID: PMC9840060
- DOI: 10.1002/prp2.1053
Comparative evaluation of biased agonists Sarcosine1 , d-Alanine8 -Angiotensin (Ang) II (SD Ang II) and Sarcosine1 , Isoleucine8 -Ang II (SI Ang II) and their radioiodinated congeners binding to rat liver membrane AT1 receptors
Abstract
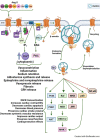
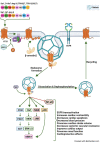
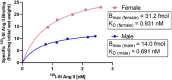
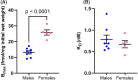
Angiotensin II analogue and β-arrestin biased agonist TRV027 (Sarcosine1 , d-Alanine8 -Angiotensin (Ang) II; SD Ang II), developed by Trevena, Inc. in the early 2010s, brought hopes of a novel treatment for cardiovascular diseases, due to its ability to simultaneously cause signaling through the β-arrestin signaling pathway, while antagonizing the pathophysiological effects of Ang II mediated by the AT1 receptor G protein signaling cascades. However, a phase II clinical trial of this agent revealed no significant benefit compared to placebo treatment. Using 125 I-Sarcosine1 , Isoleucine8 -Ang II (125 I-SI Ang II) radioligand receptor competition binding assays, we assessed the relative affinity of TRV027 compared to SI Ang II for liver AT1 receptors. We also compared radioiodinated TRV027 (125 I-SD Ang II) binding affinity for liver AT1 receptors with 125 I-SI Ang II. We found that despite its anticipated gain in metabolic stability, TRV027 and 125 I-SD Ang II had reduced affinity for the AT1 receptor compared with SI Ang II and 125 I-SI Ang II. Additionally, male-female comparisons showed that females have a higher AT1 receptor density, potentially attributed to tissue-dependent estrogen and progesterone effects. Peptide drugs have become more popular over the years due to their increased bioavailability, fast onset of action, high specificity, and low toxicity. Even though Trevena®'s biased agonist peptide TRV027 offered greater stability and potency compared to earlier AT1 R biased agonists, it failed its phase II clinical trial in 2016. Further refinements to AT1 R biased agonist peptides to improve affinity, as seen with SI Ang II, with better stability and bioavailability, has the potential to achieve the anticipated biased agonism.
Keywords: AT1R; GPCR; Sarcosine1, Isoleucine8-Angiotensin II; TRV027; binding assay; d-Alanine8-Angiotensin II.
© 2023 The Authors. Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Figures



Similar articles
-
Novel high molecular weight albumin-conjugated angiotensin II activates β-arrestin and G-protein pathways.Endocrine. 2019 Nov;66(2):349-359. doi: 10.1007/s12020-019-01930-z. Epub 2019 Apr 24. Endocrine. 2019. PMID: 31020463 Free PMC article.
-
Cardioprotective Angiotensin-(1-7) Peptide Acts as a Natural-Biased Ligand at the Angiotensin II Type 1 Receptor.Hypertension. 2016 Dec;68(6):1365-1374. doi: 10.1161/HYPERTENSIONAHA.116.08118. Epub 2016 Oct 3. Hypertension. 2016. PMID: 27698068
-
Targeting the Angiotensin II Type 1 Receptor in Cerebrovascular Diseases: Biased Signaling Raises New Hopes.Int J Mol Sci. 2021 Jun 23;22(13):6738. doi: 10.3390/ijms22136738. Int J Mol Sci. 2021. PMID: 34201646 Free PMC article. Review.
-
Influence of tissue freezing on the binding of 125I-sarcosine1, isoleucine8 angiotensin II to angiotensin II receptor subtypes in the rat.J Pharmacol Toxicol Methods. 1995 Apr;33(2):83-90. doi: 10.1016/1056-8719(94)00061-8. J Pharmacol Toxicol Methods. 1995. PMID: 7766920
-
Biased agonism of the angiotensin II type 1 receptor.Mini Rev Med Chem. 2012 Aug;12(9):812-6. doi: 10.2174/138955712800959134. Mini Rev Med Chem. 2012. PMID: 22681254 Review.
Cited by
-
Insights Into the Role of Angiotensin-II AT1 Receptor-Dependent β-Arrestin Signaling in Cardiovascular Disease.Hypertension. 2024 Jan;81(1):6-16. doi: 10.1161/HYPERTENSIONAHA.123.19419. Epub 2023 Jul 14. Hypertension. 2024. PMID: 37449411 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

