Effectiveness and safety of Jinshuibao capsules in treatment of residual cardiopulmonary symptoms in convalescent patients of coronavirus disease 2019: a pilot randomized, double-blind, placebo-controlled clinical trial
- PMID: 36640004
- PMCID: PMC9924741
- DOI: 10.19852/j.cnki.jtcm.2023.01.012
Effectiveness and safety of Jinshuibao capsules in treatment of residual cardiopulmonary symptoms in convalescent patients of coronavirus disease 2019: a pilot randomized, double-blind, placebo-controlled clinical trial
Abstract
OBJECTIVE:: To evaluate the effectiveness and safety of Jinshuibao capsules (金水宝胶囊), a Traditional Chinese Medicine (TCM), in the treatment of residual cardiopulmonary symptoms in convalescent corona virus disease 2019 (COVID-19) patients.
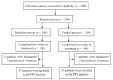
METHODS:: A total of 200 participants with COVID-19 in convalescence phase were randomly assigned into two groups at a 1:1 ratio in this multicenter randomized, double-blind, placebo-controlled trial. One group received Jinshuibao capsules, and the other received placebo. The patients were followed up at one and two weeks of treatment. Five symptoms (dry cough, shortness of breath, sweating, chest tightness and palpitation) improvement rates and full recovery rates were compared.
RESULTS:: All baseline characteristics were comparable between the two groups. After two weeks of treatment, symptom improvement rates for dry cough (74.00% vs 50.00%, P = 0.015), shortness of breath (78.95% vs 46.15%, P < 0.001), sweating (80.00% vs 57.75%, P = 0.004), chest tightness (87.06% vs 60.47%, P < 0.001) and palpitation (82.50% vs 64.56%, P = 0.010) were significantly higher in the Jinshuibao group compared with the control group. Meanwhile, Jinshuibao capsules treatment also displayed more satisfactory full recovery rates of all five symptoms (dry cough 58.00% vs 19.57%, shortness of breath 18.95% vs 7.69%, sweating 36.00% vs 19.72%, chest tightness 32.94% vs 13.95%, and palpitation 48.75% vs 29.11%) in participants with COVID-19 in convalescence phase compared with the control group (P < 0.05). No severe adverse events were reported in either group.
CONCLUSIONS:: Jinshuibao capsules have the potential to improve residual cardiopulmonary symptoms in convalescent COVID-19 patients, with few adverse events.
Keywords: COVID-19; Jinshuibao capsules; convalescence; randomized controlled trial; signs and symptoms.
Figures
References
-
- Tian FN, Ke J, . Chen J, et al. Investigation and analysis of Traditional Chinese Medicine symptoms during recovery period in patients with COVID-19. Yi Yao Dao Bao 2020; 39: 637-9.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical